Metformin induces apoptosis and inhibits migration by activating the AMPK/p53 axis and suppressing PI3K/AKT signaling in human cervical cancer cells
- PMID: 33236135
- PMCID: PMC7716426
- DOI: 10.3892/mmr.2020.11725
Metformin induces apoptosis and inhibits migration by activating the AMPK/p53 axis and suppressing PI3K/AKT signaling in human cervical cancer cells
Abstract
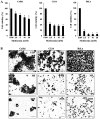
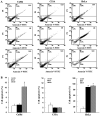
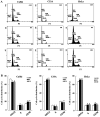
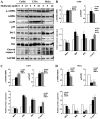
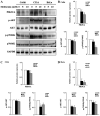
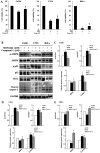
Human cervical cancer is the fourth most common malignancy among women worldwide, and it is expected to result in 460,000 deaths per year by 2040. Moreover, patients with cervical cancer often display drug resistance and severe side effects; therefore, the development of effective novel chemotherapeutic agents is important. In the present study, the effects of metformin, a first‑line therapeutic drug for type 2 diabetes mellitus, were evaluated in cervical cancer. Compared with the control group, metformin significantly inhibited cell viability and migration, and induced apoptosis and cell cycle arrest in human cervical cancer cell lines (CaSki and HeLa). Following metformin treatment, the protein expression levels of p‑AMP‑activated protein kinase (p‑AMPK), which promotes cell death, and the tumor suppressor protein p‑p53 were remarkably upregulated in CaSki and C33A cells compared with the control group. Furthermore, compared with the control group, metformin significantly suppressed the PI3K/AKT signaling pathway in CaSki, C33A and HeLa cells. Compound C (an AMPK inhibitor) significantly reversed the effects of metformin on CaSki, C33A and HeLa cell viability, and AMPK and p53 phosphorylation. The results of the present study suggested that metformin induced AMPK‑mediated apoptosis, thus metformin may serve as a chemotherapeutic agent for human cervical cancer.
Keywords: metformin; apoptosis; migration; AMPK‑activated protein kinase/p53; PI3K/AKT; cervical cancer.
Figures
Similar articles
-
Metformin inhibits cervical cancer cell proliferation by modulating PI3K/Akt-induced major histocompatibility complex class I-related chain A gene expression.J Exp Clin Cancer Res. 2020 Jul 6;39(1):127. doi: 10.1186/s13046-020-01627-6. J Exp Clin Cancer Res. 2020. PMID: 32631421 Free PMC article.
-
miR‑519d‑3p/HIF‑2α axis increases the chemosensitivity of human cervical cancer cells to cisplatin via inactivation of PI3K/AKT signaling.Mol Med Rep. 2021 May;23(5):353. doi: 10.3892/mmr.2021.11992. Epub 2021 Mar 24. Mol Med Rep. 2021. PMID: 33760204
-
LY294002 and metformin cooperatively enhance the inhibition of growth and the induction of apoptosis of ovarian cancer cells.Int J Gynecol Cancer. 2012 Jan;22(1):15-22. doi: 10.1097/IGC.0b013e3182322834. Int J Gynecol Cancer. 2012. PMID: 22080879
-
PI3K/Akt-mediated regulation of p53 in cancer.Biochem Soc Trans. 2014 Aug;42(4):798-803. doi: 10.1042/BST20140070. Biochem Soc Trans. 2014. PMID: 25109960 Review.
-
New light on treatment of cervical cancer: Chinese medicine monomers can be effective for cervical cancer by inhibiting the PI3K/Akt signaling pathway.Biomed Pharmacother. 2023 Jan;157:114084. doi: 10.1016/j.biopha.2022.114084. Epub 2022 Dec 5. Biomed Pharmacother. 2023. PMID: 36481407 Review.
Cited by
-
Anti-Proliferative Properties of the Novel Hybrid Drug Met-ITC, Composed of the Native Drug Metformin with the Addition of an Isothiocyanate H2S Donor Moiety, in Different Cancer Cell Lines.Int J Mol Sci. 2023 Nov 9;24(22):16131. doi: 10.3390/ijms242216131. Int J Mol Sci. 2023. PMID: 38003321 Free PMC article.
-
SIRT2 inhibitor SirReal2 enhances anti-tumor effects of PI3K/mTOR inhibitor VS-5584 on acute myeloid leukemia cells.Cancer Med. 2023 Sep;12(18):18901-18917. doi: 10.1002/cam4.6480. Epub 2023 Sep 1. Cancer Med. 2023. PMID: 37658623 Free PMC article.
-
Effect of metformin on irinotecan-induced cell cycle arrest in colorectal cancer cell lines HCT116 and SW480.Int J Health Sci (Qassim). 2021 Sep-Oct;15(5):34-41. Int J Health Sci (Qassim). 2021. PMID: 34548861 Free PMC article.
-
The development and benefits of metformin in various diseases.Front Med. 2023 Jun;17(3):388-431. doi: 10.1007/s11684-023-0998-6. Epub 2023 Jul 4. Front Med. 2023. PMID: 37402952 Review.
-
Network Pharmacology and Intestinal Microbiota Analysis Revealing the Mechanism of Punicalagin Improving Bacterial Enteritis.Curr Comput Aided Drug Des. 2024;20(2):104-120. doi: 10.2174/1573409919666230526165501. Curr Comput Aided Drug Des. 2024. PMID: 37246319 Free PMC article.
References
-
- Simms KT, Steinberg J, Caruana M, Smith MA, Lew JB, Soerjomataram I, Castle PE, Bray F, Canfell K. Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020-99: A modelling study. Lancet Oncol. 2019;20:394–407. doi: 10.1016/S1470-2045(18)30836-2. - DOI - PubMed
-
- Rosen VM, Guerra I, McCormack M, Nogueira-Rodrigues A, Sasse A, Munk VC, Shang A. Systematic review and network meta-analysis of bevacizumab plus first-line topotecan-paclitaxel or cisplatin-paclitaxel versus non-bevacizumab-containing therapies in persistent, recurrent, or metastatic cervical cancer. Int J Gynecol Cancer. 2017;27:1237–1246. doi: 10.1097/IGC.0000000000001000. - DOI - PMC - PubMed
-
- Cervical Cancer. National Cancer Institute; May, 2020. Bethesda. SEER Cancer Stat Facts.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

