IL3RA-Targeting Antibody-Drug Conjugate BAY-943 with a Kinesin Spindle Protein Inhibitor Payload Shows Efficacy in Preclinical Models of Hematologic Malignancies
- PMID: 33233768
- PMCID: PMC7709048
- DOI: 10.3390/cancers12113464
IL3RA-Targeting Antibody-Drug Conjugate BAY-943 with a Kinesin Spindle Protein Inhibitor Payload Shows Efficacy in Preclinical Models of Hematologic Malignancies
Abstract
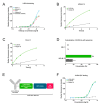
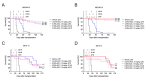
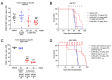
IL3RA (CD123) is the alpha subunit of the interleukin 3 (IL-3) receptor, which regulates the proliferation, survival, and differentiation of hematopoietic cells. IL3RA is frequently expressed in acute myeloid leukemia (AML) and classical Hodgkin lymphoma (HL), presenting an opportunity to treat AML and HL with an IL3RA-directed antibody-drug conjugate (ADC). Here, we describe BAY-943 (IL3RA-ADC), a novel IL3RA-targeting ADC consisting of a humanized anti-IL3RA antibody conjugated to a potent proprietary kinesin spindle protein inhibitor (KSPi). In vitro, IL3RA-ADC showed potent and selective antiproliferative efficacy in a panel of IL3RA-expressing AML and HL cell lines. In vivo, IL3RA-ADC improved survival and reduced tumor burden in IL3RA-positive human AML cell line-derived (MOLM-13 and MV-4-11) as well as in patient-derived xenograft (PDX) models (AM7577 and AML11655) in mice. Furthermore, IL3RA-ADC induced complete tumor remission in 12 out of 13 mice in an IL3RA-positive HL cell line-derived xenograft model (HDLM-2). IL3RA-ADC was well-tolerated and showed no signs of thrombocytopenia, neutropenia, or liver toxicity in rats, or in cynomolgus monkeys when dosed up to 20 mg/kg. Overall, the preclinical results support the further development of BAY-943 as an innovative approach for the treatment of IL3RA-positive hematologic malignancies.
Keywords: CD123; IL3RA; acute myeloid leukemia; antibody-drug conjugate; kinesin spindle protein inhibitor.
Conflict of interest statement
All authors are current or former employees of Bayer AG and inventors on Bayer AG patent applications. Anette Sommer, Hans-Georg Lerchen, Stephan Märsch, Michael Erkelenz, and Dominik Mumberg have ownership interest as shares in Bayer AG.
Figures

Similar articles
-
Development of Highly Optimized Antibody-Drug Conjugates against CD33 and CD123 for Acute Myeloid Leukemia.Clin Cancer Res. 2021 Jan 15;27(2):622-631. doi: 10.1158/1078-0432.CCR-20-2149. Epub 2020 Nov 4. Clin Cancer Res. 2021. PMID: 33148666
-
A CD123-targeting antibody-drug conjugate, IMGN632, designed to eradicate AML while sparing normal bone marrow cells.Blood Adv. 2018 Apr 24;2(8):848-858. doi: 10.1182/bloodadvances.2018017517. Blood Adv. 2018. PMID: 29661755 Free PMC article.
-
Preclinical Antitumor Efficacy of BAY 1129980-a Novel Auristatin-Based Anti-C4.4A (LYPD3) Antibody-Drug Conjugate for the Treatment of Non-Small Cell Lung Cancer.Mol Cancer Ther. 2017 May;16(5):893-904. doi: 10.1158/1535-7163.MCT-16-0474. Epub 2017 Mar 14. Mol Cancer Ther. 2017. PMID: 28292941
-
Combining Biology and Chemistry for a New Take on Chemotherapy: Antibody-Drug Conjugates in Hematologic Malignancies.Curr Hematol Malig Rep. 2018 Dec;13(6):555-569. doi: 10.1007/s11899-018-0485-3. Curr Hematol Malig Rep. 2018. PMID: 30362019 Review.
-
CD123 as a Therapeutic Target in the Treatment of Hematological Malignancies.Cancers (Basel). 2019 Sep 12;11(9):1358. doi: 10.3390/cancers11091358. Cancers (Basel). 2019. PMID: 31547472 Free PMC article. Review.
Cited by
-
Novel Agents For Relapsed and Refractory Classical Hodgkin Lymphoma: A Review.Front Oncol. 2022 Jul 14;12:929012. doi: 10.3389/fonc.2022.929012. eCollection 2022. Front Oncol. 2022. PMID: 35928877 Free PMC article. Review.
-
Network controllability solutions for computational drug repurposing using genetic algorithms.Sci Rep. 2022 Jan 26;12(1):1437. doi: 10.1038/s41598-022-05335-3. Sci Rep. 2022. PMID: 35082323 Free PMC article.
-
Antibody-drug conjugates harboring a kinesin spindle protein inhibitor with immunostimulatory properties.Oncoimmunology. 2022 Feb 9;11(1):2037216. doi: 10.1080/2162402X.2022.2037216. eCollection 2022. Oncoimmunology. 2022. PMID: 35154909 Free PMC article.
-
Antibody-drug conjugates for the treatment of lymphoma: clinical advances and latest progress.J Hematol Oncol. 2021 Jun 5;14(1):88. doi: 10.1186/s13045-021-01097-z. J Hematol Oncol. 2021. PMID: 34090506 Free PMC article. Review.
-
Neutrophil elastase as a versatile cleavage enzyme for activation of αvβ3 integrin-targeted small molecule drug conjugates with different payload classes in the tumor microenvironment.Front Pharmacol. 2024 Mar 1;15:1358393. doi: 10.3389/fphar.2024.1358393. eCollection 2024. Front Pharmacol. 2024. PMID: 38495100 Free PMC article.
References
-
- Ehninger A., Kramer M., Röllig C., Thiede C., Bornhäuser M., von Bonin M., Wermke M., Feldmann A., Bachmann M., Ehninger G., et al. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J. 2014;4:e218. doi: 10.1038/bcj.2014.39. - DOI - PMC - PubMed
-
- Muñoz L., Nomdedéu J.F., Lopez O., Carnicer M.J., Bellido M., Aventín A., Brunet S., Sierra J. Interleukin-3 receptor alpha chain (CD123) is widely expressed in hematologic malignancies. Haematologica. 2001;86:1261–1269. - PubMed
-
- Bras A.E., de Haas V., van Stigt A., Jongen-Lavrencic M., Beverloo H.B., Te Marvelde J.G., Zwaan C.M., van Dongen J.J.M., Leusen J.H.W., van der Velden V.H.J. CD123 expression levels in 846 acute leukemia patients based on standardized immunophenotyping. Cytom. Part B Clin. Cytom. 2019;96:134–142. doi: 10.1002/cyto.b.21745. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

