Potential Nutraceutical Properties of Leaves from Several Commonly Cultivated Plants
- PMID: 33203123
- PMCID: PMC7698063
- DOI: 10.3390/biom10111556
Potential Nutraceutical Properties of Leaves from Several Commonly Cultivated Plants
Abstract
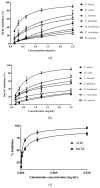
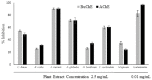
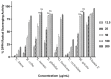
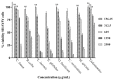
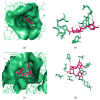
Chronic dietary ingestion of suitable phytochemicals may assist with limiting or negating neurodegenerative decline. Current therapeutics used to treat Alzheimer disease elicit broad adverse drug reactions, and alternative sources of cholinesterase inhibitors (ChEIs) are required. Herein, we screened methanolic extracts from seven commonly cultivated plants for their nutraceutical potential; ability to inhibit acetylcholinesterase (AChE) and butyryl-cholinesterase (BuChE), and provision of antioxidant activity through their 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) free radical scavenging capabilities. Potential neurotoxicity of plant extracts was examined via application to SHSY-5Y neuroblastoma cells and quantitation of cell viability. Methanolic extracts of Citrus limon (Lemon), Bombax ceiba (Red silk-cotton), Lawsonia inermis (Henna), Eucalyptus globulus (Eucalyptus), Ocimum basilicum (Basil), Citrus reticulata (Mandarin orange), and Mentha spicata (Spearmint) all displayed concentration-dependent inhibition of AChE and BuChE. The majority of extracts inhibited AChE and BuChE to near equipotency, with Henna and Eucalyptus extracts the two most potent ChEIs. All plant extracts were able to scavenge free radicals in a concentration-dependent manner, with Eucalyptus the most potent antioxidant. Toxicity of plant extracts to neuronal cells was concentration dependent, with Eucalyptus also the most toxic extract. Fractionation of plant extracts and analysis by mass spectrometry identified a number of plant polyphenols that might have contributed to the cholinesterase inhibition: 3-caffeoylquinic acid, methyl 4-caffeoylquinate, kaempferol-acetyl-glycoside, quercetin 3-rutinoside, quercetin-acetyl-glycoside, kaempferol 3-O-glucoside, and quercetin 3-O-glucoside. In silico molecular modeling of these polyphenols demonstrated their improved AChE and BuChE binding affinities compared to the current FDA-approved dual ChEI, galantamine. Collectively, all the plant extracts contained nutraceutical agents as antioxidants and ChEIs and, therefore, their chronic consumption may prove beneficial to combat the pathological deficits that accrue in Alzheimer disease.
Keywords: Alzheimer disease; acetylcholinesterase inhibitors; antioxidants; butyrylcholinesterase inhibitors; molecular modelling; nutraceuticals; phytochemicals.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Evaluation of anti-amnesic effect of extracts of selected Ocimum species using in-vitro and in-vivo models.J Ethnopharmacol. 2016 Dec 4;193:490-499. doi: 10.1016/j.jep.2016.10.026. Epub 2016 Oct 8. J Ethnopharmacol. 2016. PMID: 27725240
-
Phytochemical profiling and in vitro screening for anticholinesterase, antioxidant, antiglucosidase and neuroprotective effect of three traditional medicinal plants for Alzheimer's Disease and Diabetes Mellitus dual therapy.BMC Complement Altern Med. 2018 Mar 2;18(1):77. doi: 10.1186/s12906-018-2140-x. BMC Complement Altern Med. 2018. PMID: 29499679 Free PMC article.
-
Cholinesterase inhibitory, anti-amyloidogenic and neuroprotective effect of the medicinal plant Grewia tiliaefolia - An in vitro and in silico study.Pharm Biol. 2017 Dec;55(1):381-393. doi: 10.1080/13880209.2016.1241811. Pharm Biol. 2017. PMID: 27931177 Free PMC article.
-
Determination of Anti-Alzheimer's Disease Activity of Selected Plant Ingredients.Molecules. 2022 May 18;27(10):3222. doi: 10.3390/molecules27103222. Molecules. 2022. PMID: 35630702 Free PMC article. Review.
-
The role of phytochemicals in the treatment and prevention of dementia.Drugs Aging. 2011 Jun 1;28(6):439-68. doi: 10.2165/11591310-000000000-00000. Drugs Aging. 2011. PMID: 21639405 Review.
Cited by
-
Phytochemical Analysis and Evaluation of Biological Activity of Lawsonia inermis Seeds Related to Alzheimer's Disease.Evid Based Complement Alternat Med. 2021 Jul 19;2021:5965061. doi: 10.1155/2021/5965061. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34335830 Free PMC article.
-
Anti-Cholinesterase Combination Drug Therapy as a Potential Treatment for Alzheimer's Disease.Brain Sci. 2021 Feb 2;11(2):184. doi: 10.3390/brainsci11020184. Brain Sci. 2021. PMID: 33540879 Free PMC article.
-
Advantages of Spray Drying over Freeze Drying: A Comparative Analysis of Lonicera caerulea L. Juice Powders-Matrix Diversity and Bioactive Response.Molecules. 2024 Jul 30;29(15):3586. doi: 10.3390/molecules29153586. Molecules. 2024. PMID: 39124991 Free PMC article.
-
Nuts as functional foods: Variation of nutritional and phytochemical profiles and their in vitro bioactive properties.Food Chem X. 2022 Aug 8;15:100418. doi: 10.1016/j.fochx.2022.100418. eCollection 2022 Oct 30. Food Chem X. 2022. PMID: 36211787 Free PMC article.
-
An Integrated NMR, LC-DAD-MS, LC-QTOF Metabolomic Characterization of Sartoria hedysaroides: Correlation of Antioxidant and Enzyme Inhibitory Activity with Chemical Composition by Multivariate Data Analysis.Antioxidants (Basel). 2022 Jan 4;11(1):110. doi: 10.3390/antiox11010110. Antioxidants (Basel). 2022. PMID: 35052614 Free PMC article.
References
-
- United Nations Department of Economic and Social Affairs, Population Division World Population Ageing 2017—Highlights (ST/ESA/SER.A/397) [(accessed on 14 November 2020)]; Available online: https://www.un.org/en/development/desa/population/publications/pdf/agein....
-
- WHO . WHO Media Centre; 2016. [(accessed on 28 September 2020)]. 10 Facts on Dementia. Available online: https://www.who.int/features/factfiles/dementia/en/
-
- Launer L.J., Andersen K., Dewey M.E., Letenneur L., Ott A., Amaducci L.A., Brayne C.E., Copeland J.R.M., Dartigues J.F., Kragh-Sorensen P., et al. Rates and risk factors for dementia and Alzheimer’s disease: Results from EURODEM pooled analyses. Neurology. 1999;52:78. doi: 10.1212/WNL.52.1.78. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

