Improving target assessment in biomedical research: the GOT-IT recommendations
- PMID: 33199880
- PMCID: PMC7667479
- DOI: 10.1038/s41573-020-0087-3
Improving target assessment in biomedical research: the GOT-IT recommendations
Abstract
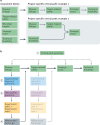
Academic research plays a key role in identifying new drug targets, including understanding target biology and links between targets and disease states. To lead to new drugs, however, research must progress from purely academic exploration to the initiation of efforts to identify and test a drug candidate in clinical trials, which are typically conducted by the biopharma industry. This transition can be facilitated by a timely focus on target assessment aspects such as target-related safety issues, druggability and assayability, as well as the potential for target modulation to achieve differentiation from established therapies. Here, we present recommendations from the GOT-IT working group, which have been designed to support academic scientists and funders of translational research in identifying and prioritizing target assessment activities and in defining a critical path to reach scientific goals as well as goals related to licensing, partnering with industry or initiating clinical development programmes. Based on sets of guiding questions for different areas of target assessment, the GOT-IT framework is intended to stimulate academic scientists' awareness of factors that make translational research more robust and efficient, and to facilitate academia-industry collaboration.
Conflict of interest statement
M.J.P. is a member of the Scientific Advisory Boards of EpiEndo Pharmaceuticals, Cyclone Therapeutics and aidCURE GmbH. A.B. is an employee and/or shareholder of PAASP GmbH, PAASP US LLC, EXCIVA GmbH, Synventa LLC and RITEC Pharma. The other authors declare no competing financial interests.
Figures

Similar articles
-
Australia in 2030: what is our path to health for all?Med J Aust. 2021 May;214 Suppl 8:S5-S40. doi: 10.5694/mja2.51020. Med J Aust. 2021. PMID: 33934362
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
Dynamic Field Theory of Executive Function: Identifying Early Neurocognitive Markers.Monogr Soc Res Child Dev. 2024 Dec;89(3):7-109. doi: 10.1111/mono.12478. Monogr Soc Res Child Dev. 2024. PMID: 39628288 Free PMC article.
-
Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
-
Factors Determining Plasticity of Responses to Drugs.Int J Mol Sci. 2022 Feb 13;23(4):2068. doi: 10.3390/ijms23042068. Int J Mol Sci. 2022. PMID: 35216184 Free PMC article. Review.
-
Proteomic Data Advance Targeted Drug Development for Neurogenerative Diseases.Biol Psychiatry. 2023 May 1;93(9):754-755. doi: 10.1016/j.biopsych.2023.02.003. Biol Psychiatry. 2023. PMID: 37045511 Free PMC article. No abstract available.
-
Recommendations for robust and reproducible preclinical research in personalised medicine.BMC Med. 2023 Jan 8;21(1):14. doi: 10.1186/s12916-022-02719-0. BMC Med. 2023. PMID: 36617553 Free PMC article. Review.
-
Unraveling the Mechanism of Epichaperome Modulation by Zelavespib: Biochemical Insights on Target Occupancy and Extended Residence Time at the Site of Action.Biomedicines. 2023 Sep 22;11(10):2599. doi: 10.3390/biomedicines11102599. Biomedicines. 2023. PMID: 37892973 Free PMC article.
-
Predicting drug targets by homology modelling of Pseudomonas aeruginosa proteins of unknown function.PLoS One. 2021 Oct 14;16(10):e0258385. doi: 10.1371/journal.pone.0258385. eCollection 2021. PLoS One. 2021. PMID: 34648550 Free PMC article.
References
-
- Bunnage ME. Getting pharmaceutical R&D back on target. Nat. Chem. Biol. 2011;7:335–339. - PubMed
-
- Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 2014;32:40–51. - PubMed
-
- Dowden H, Munro J. Trends in clinical success rates and therapeutic focus. Nat. Rev. Drug Discov. 2019;18:495–496. - PubMed
-
- Paul SM, et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010;9:203–214. - PubMed
-
- Blake, R. A. in High Content Screening: A Powerful Approach to Systems Cell Biology and Drug Discovery (eds Taylor, D. L., Haskins, J. R. & Giuliano, K. A.) 367–377 (Humana, 2006).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

