Inflammatory Cytokines Alter Mesenchymal Stem Cell Mechanosensing and Adhesion on Stiffened Infarct Heart Tissue After Myocardial Infarction
- PMID: 33195229
- PMCID: PMC7645114
- DOI: 10.3389/fcell.2020.583700
Inflammatory Cytokines Alter Mesenchymal Stem Cell Mechanosensing and Adhesion on Stiffened Infarct Heart Tissue After Myocardial Infarction
Abstract
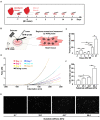
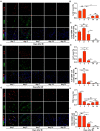
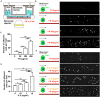
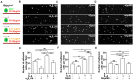
Mesenchymal stem cell (MSC) transplantation has demonstrated its potential in repairing infarct heart tissue and recovering heart function after myocardial infarction (MI). However, its therapeutic effect is still limited due to poor MSC engraftment at the injury site whose tissue stiffness and local inflammation both dynamically and rapidly change after MI. Whether and how inflammatory cytokines could couple with stiffness change to affect MSC engraftment in the infarct zone still remain unclear. In this study, we characterized dynamic stiffness changes of and inflammatory cytokine expression in the infarct region of rat heart within a month after MI. We found that the tissue stiffness of the heart tissue gradually increased and peaked 21 days after MI along with the rapid upregulation of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) in the first 3 days, followed by a sharp decline. We further demonstrated in vitro that immobilized inflammatory cytokine IL-6 performed better than the soluble form in enhancing MSC adhesion to stiffened substrate through IL-6/src homology 2 (SH2) domain-containing tyrosine phosphatase-2 (SHP2)/integrin signaling axis. We also confirmed such mechano-immune coupling of tissue stiffness and inflammatory cytokines in modulating MSC engraftment in the rat heart after MI in vivo. Our study provides new mechanistic insights of mechanical-inflammation coupling to improve MSC mechanosensing and adhesion, potentially benefiting MSC engraftment and its clinical therapy for MI.
Keywords: adhesion; cytokine; mechanosensing; mesenchymal stem cell; myocardial infarction.
Copyright © 2020 Zhu, Wu, Xiao, Hu, Zhang, Hu, Chen and Wang.
Figures
Similar articles
-
Immuno-inflammatory regulation effect of mesenchymal stem cell transplantation in a rat model of myocardial infarction.Cytotherapy. 2008;10(5):469-78. doi: 10.1080/14653240802129893. Cytotherapy. 2008. PMID: 18608353
-
Functional and bioenergetic modulations in the infarct border zone following autologous mesenchymal stem cell transplantation.Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1772-80. doi: 10.1152/ajpheart.00242.2007. Epub 2007 Jun 15. Am J Physiol Heart Circ Physiol. 2007. PMID: 17573463
-
Macrophage subpopulations are essential for infarct repair with and without stem cell therapy.J Am Coll Cardiol. 2013 Nov 12;62(20):1890-901. doi: 10.1016/j.jacc.2013.07.057. Epub 2013 Aug 21. J Am Coll Cardiol. 2013. PMID: 23973704
-
Extracardiac-Lodged Mesenchymal Stromal Cells Propel an Inflammatory Response Against Myocardial Infarction via Paracrine Effects.Cell Transplant. 2016;25(5):929-35. doi: 10.3727/096368915X689758. Epub 2015 Oct 22. Cell Transplant. 2016. PMID: 26498018 Review.
-
Can the outcomes of mesenchymal stem cell-based therapy for myocardial infarction be improved? Providing weapons and armour to cells.Cell Prolif. 2017 Apr;50(2):e12316. doi: 10.1111/cpr.12316. Epub 2016 Nov 23. Cell Prolif. 2017. PMID: 27878916 Free PMC article. Review.
Cited by
-
Inflammation in myocardial infarction: roles of mesenchymal stem cells and their secretome.Cell Death Discov. 2022 Nov 9;8(1):452. doi: 10.1038/s41420-022-01235-7. Cell Death Discov. 2022. PMID: 36351896 Free PMC article. Review.
-
A 3D Platform to Investigate Dynamic Cell-to-Cell Interactions Between Tumor Cells and Mesenchymal Progenitors.Front Cell Dev Biol. 2022 Jan 17;9:767253. doi: 10.3389/fcell.2021.767253. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35111750 Free PMC article.
-
peri-Adventitial delivery of smooth muscle cells in porous collagen scaffolds for treatment of experimental abdominal aortic aneurysm.Biomater Sci. 2021 Oct 12;9(20):6903-6914. doi: 10.1039/d1bm00685a. Biomater Sci. 2021. PMID: 34522940 Free PMC article.
References
-
- Alon R., Cahalon L., Hershkoviz R., Elbaz D., Reizis B., Wallach D., et al. (1994). TNF-alpha binds to the N-terminal domain of fibronectin and augments the beta 1-integrin-mediated adhesion of CD4+ T lymphocytes to the glycoprotein. J. Immunol. 152 1304–1313. - PubMed
-
- Arunachalam S. P., Arani A., Baffour F., Rysavy J. A., Rossman P. J., Glaser K. J., et al. (2018). Regional assessment of in vivo myocardial stiffness using 3D magnetic resonance elastography in a porcine model of myocardial infarction. Magn. Reson. Med. 79 361–369. 10.1002/mrm.26695 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

