Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine
- PMID: 33194810
- PMCID: PMC7642463
- DOI: 10.3389/fcimb.2020.572681
Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine
Abstract
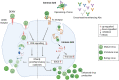
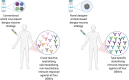
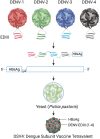
In 2019, the United States Food and Drug Administration accorded restricted approval to Sanofi Pasteur's Dengvaxia, a live attenuated vaccine (LAV) for dengue fever, a mosquito-borne viral disease, caused by four antigenically distinct dengue virus serotypes (DENV 1-4). The reason for this limited approval is the concern that this vaccine sensitized some of the dengue-naïve recipients to severe dengue fever. Recent knowledge about the nature of the immune response elicited by DENV viruses suggests that all LAVs have inherent capacity to predominantly elicit antibodies (Abs) against the pre-membrane (prM) and fusion loop epitope (FLE) of DENV. These antibodies are generally cross-reactive among DENV serotypes carrying a higher risk of promoting Antibody-Dependent Enhancement (ADE). ADE is a phenomenon in which suboptimal neutralizing or non-neutralizing cross-reactive antibodies bind to virus and facilitate Fcγ receptor mediated enhanced entry into host cells, followed by its replication, and thus increasing the cellular viral load. On the other hand, antibody responses directed against the host-cell receptor binding domain of DENV envelope domain-III (EDIII), exhibit a higher degree of type-specificity with lower potential of ADE. The challenges associated with whole DENV-based vaccine strategies necessitate re-focusing our attention toward the designed dengue vaccine candidates, capable of inducing predominantly type-specific immune responses. If the designed vaccines elicited predominantly EDIII-directed serotype specific antibodies in the absence of prM and FLE antibodies, this could avoid the ADE phenomenon largely associated with the prM and FLE antibodies. The generation of type-specific antibodies to each of the four DENV serotypes by the designed vaccines could avoid the immune evasion mechanisms of DENVs. For the enhanced vaccine safety, all dengue vaccine candidates should be assessed for the extent of type-specific (minimal ADE) vs. cross-reactive (ADE promoting) neutralizing antibodies. The type-specific EDIII antibodies may be more directly related to protection from disease in the absence of ADE promoted by the cross-reactive antibodies.
Keywords: Dengvaxia; antibody-dependent enhancement (ADE); dengue; dengue vaccine; dengue virus (DENV); live attenuated vaccine; virus-like particle.
Copyright © 2020 Shukla, Ramasamy, Shanmugam, Ahuja and Khanna.
Figures

Similar articles
-
Dengue and Zika virus infections are enhanced by live attenuated dengue vaccine but not by recombinant DSV4 vaccine candidate in mouse models.EBioMedicine. 2020 Oct;60:102991. doi: 10.1016/j.ebiom.2020.102991. Epub 2020 Sep 16. EBioMedicine. 2020. PMID: 32949997 Free PMC article.
-
Virus-like particles derived from Pichia pastoris-expressed dengue virus type 1 glycoprotein elicit homotypic virus-neutralizing envelope domain III-directed antibodies.BMC Biotechnol. 2016 Jun 14;16(1):50. doi: 10.1186/s12896-016-0280-y. BMC Biotechnol. 2016. PMID: 27301568 Free PMC article.
-
Pichia pastoris-Expressed Bivalent Virus-Like Particulate Vaccine Induces Domain III-Focused Bivalent Neutralizing Antibodies without Antibody-Dependent Enhancement in Vivo.Front Microbiol. 2018 Jan 9;8:2644. doi: 10.3389/fmicb.2017.02644. eCollection 2017. Front Microbiol. 2018. PMID: 29367852 Free PMC article.
-
Modulation of Dengue/Zika Virus Pathogenicity by Antibody-Dependent Enhancement and Strategies to Protect Against Enhancement in Zika Virus Infection.Front Immunol. 2018 Apr 23;9:597. doi: 10.3389/fimmu.2018.00597. eCollection 2018. Front Immunol. 2018. PMID: 29740424 Free PMC article. Review.
-
Paradoxical role of antibodies in dengue virus infections: considerations for prophylactic vaccine development.Expert Rev Vaccines. 2016;15(4):467-82. doi: 10.1586/14760584.2016.1121814. Epub 2015 Dec 15. Expert Rev Vaccines. 2016. PMID: 26577689 Review.
Cited by
-
Association of dengue infection with anti-alpha-gal antibodies, IgM, IgG, IgG1, and IgG2.Front Immunol. 2022 Oct 14;13:1021016. doi: 10.3389/fimmu.2022.1021016. eCollection 2022. Front Immunol. 2022. PMID: 36311743 Free PMC article.
-
Outsmarting Pathogens with Antibody Engineering.Annu Rev Chem Biomol Eng. 2023 Jun 8;14:217-241. doi: 10.1146/annurev-chembioeng-101121-084508. Epub 2023 Mar 14. Annu Rev Chem Biomol Eng. 2023. PMID: 36917814 Free PMC article. Review.
-
Innate Immune Response to Dengue Virus: Toll-like Receptors and Antiviral Response.Viruses. 2022 May 7;14(5):992. doi: 10.3390/v14050992. Viruses. 2022. PMID: 35632732 Free PMC article. Review.
-
A tetravalent nanoparticle vaccine elicits a balanced and potent immune response against dengue viruses without inducing antibody-dependent enhancement.Front Immunol. 2023 May 19;14:1193175. doi: 10.3389/fimmu.2023.1193175. eCollection 2023. Front Immunol. 2023. PMID: 37275868 Free PMC article.
-
Development and Characterization of a Genetically Stable Infectious Clone for a Genotype I Isolate of Dengue Virus Serotype 1.Viruses. 2022 Sep 18;14(9):2073. doi: 10.3390/v14092073. Viruses. 2022. PMID: 36146879 Free PMC article.
References
-
- Arredondo-García J. L., Hadinegoro S. R., Reynales H., Chua M. N., Rivera Medina D. M., Chotpitayasunondh T., et al. . (2018). Four-year safety follow-up of the tetravalent dengue vaccine efficacy randomized controlled trials in Asia and Latin America. Clin. Microbiol. Infect. 24, 755–763. 10.1016/j.cmi.2018.01.018 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

