Deletion of FUNDC2 and CMC4 on Chromosome Xq28 Is Sufficient to Cause Hypergonadotropic Hypogonadism in Men
- PMID: 33193636
- PMCID: PMC7537572
- DOI: 10.3389/fgene.2020.557341
Deletion of FUNDC2 and CMC4 on Chromosome Xq28 Is Sufficient to Cause Hypergonadotropic Hypogonadism in Men
Abstract
Background: Hypergonadotropic hypogonadism (HH) is characterized by low sex steroid levels and secondarily elevated gonadotropin levels with either congenital or acquired etiology. Genetic factors leading to HH have yet to be fully elucidated.
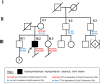
Methods: Here, we report on genome and transcriptome data analyses from a male patient with HH and history of growth delay who has an inherited deletion of chromosome Xq28. Expression analyses were done for this patient and his unaffected family members and compared to normal controls to identify dysregulated genes due to this deletion.
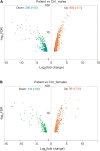
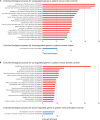
Results: Our patient's Xq28 deletion is 44,806 bp and contains only two genes, FUNDC2 and CMC4. Expression of both FUNDC2 and CMC4 are completely abolished in the patient. Gene ontology analyses of differentially expressed genes (DEGs) in the patient in comparison to controls show that significantly up-regulated genes in the patient are enriched in Sertoli cell barrier (SCB) regulation, apoptosis, inflammatory response, and gonadotropin-releasing regulation. Indeed, our patient has an elevated follicle stimulating hormone (FSH) level, which regulates Sertoli cell proliferation and spermatogenesis. In his mother and sister, who are heterozygous for this deletion, X-chromosome inactivation (XCI) is skewed toward the deleted X, suggesting a mechanism to avoid FSH dysregulation.
Conclusion: Compared to the previously reported men with variable sized Xq28 deletions, our study suggests that loss of function of FUNDC2 and CMC4 results in dysregulation of apoptosis, inflammation, and FSH, and is sufficient to cause Xq28-associated HH.
Keywords: CMC4; FUNDC2; Sertoli cell barrier; Xq28 deletion; apoptosis; hypergonadotropic hypogonadism.
Copyright © 2020 Deng, Fang, Pathak, Zou, Neufeld-Kaiser, Malouf, Failor, Hisama and Liu.
Figures

Similar articles
-
Optical genome mapping with genome sequencing identifies subtelomeric Xq28 deletion and inserted 7p22.3 duplication in a male with multisystem developmental disorder.Am J Med Genet A. 2024 Dec;194(12):e63814. doi: 10.1002/ajmg.a.63814. Epub 2024 Jul 16. Am J Med Genet A. 2024. PMID: 39011850
-
Causes of hypogonadotropic hypogonadism predict response to gonadotropin substitution in adults.Andrology. 2016 Jan;4(1):87-94. doi: 10.1111/andr.12128. Andrology. 2016. PMID: 26779870
-
The presence of two rare genomic syndromes, 1q21 deletion and Xq28 duplication, segregating independently in a family with intellectual disability.Mol Cytogenet. 2016 Sep 29;9:74. doi: 10.1186/s13039-016-0286-0. eCollection 2016. Mol Cytogenet. 2016. PMID: 27708714 Free PMC article.
-
[Stimulation of spermatogenesis in men with hypogonadotropic hypogonadism].Pol Merkur Lekarski. 2018 Sep 21;45(267):126-130. Pol Merkur Lekarski. 2018. PMID: 30240383 Review. Polish.
-
Hormonal control of spermatogenesis in men: therapeutic aspects in hypogonadotropic hypogonadism.Ann Endocrinol (Paris). 2014 May;75(2):98-100. doi: 10.1016/j.ando.2014.04.002. Epub 2014 Apr 29. Ann Endocrinol (Paris). 2014. PMID: 24793994 Review.
Cited by
-
JMJD3 regulate H3K27me3 modification via interacting directly with TET1 to affect spermatogonia self-renewal and proliferation.BMC Genomics. 2024 Feb 29;25(1):225. doi: 10.1186/s12864-024-10120-9. BMC Genomics. 2024. PMID: 38424516 Free PMC article.
-
Large-scale analyses of the X chromosome in 2,354 infertile men discover recurrently affected genes associated with spermatogenic failure.Am J Hum Genet. 2022 Aug 4;109(8):1458-1471. doi: 10.1016/j.ajhg.2022.06.007. Epub 2022 Jul 8. Am J Hum Genet. 2022. PMID: 35809576 Free PMC article.
-
Illuminating the terminal nerve: Uncovering the link between GnRH-1 neuron and olfactory development.J Comp Neurol. 2024 Mar;532(3):e25599. doi: 10.1002/cne.25599. J Comp Neurol. 2024. PMID: 38488687 Free PMC article.
-
The Genetic Etiology Diagnosis of Fetal Growth Restriction Using Single-Nucleotide Polymorphism-Based Chromosomal Microarray Analysis.Front Pediatr. 2021 Oct 15;9:743639. doi: 10.3389/fped.2021.743639. eCollection 2021. Front Pediatr. 2021. PMID: 34722424 Free PMC article.
-
FUNDC2, a mitochondrial outer membrane protein, mediates triple-negative breast cancer progression via the AKT/GSK3β/GLI1 pathway.Acta Biochim Biophys Sin (Shanghai). 2023 Nov 25;55(11):1770-1783. doi: 10.3724/abbs.2023142. Acta Biochim Biophys Sin (Shanghai). 2023. PMID: 37700593 Free PMC article.
References
-
- Basaria S. (2014). Male hypogonadism. Lancet 383 1250–1263. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

