Much More Than a Cytoskeletal Protein: Physiological and Pathological Functions of the Non-microtubule Binding Region of Tau
- PMID: 33193056
- PMCID: PMC7604284
- DOI: 10.3389/fneur.2020.590059
Much More Than a Cytoskeletal Protein: Physiological and Pathological Functions of the Non-microtubule Binding Region of Tau
Abstract
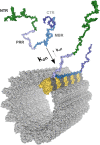
Tau protein (MAPT) is classified as a microtubule-associated protein (MAP) and is believed to regulate the axonal microtubule arrangement. It belongs to the tau/MAP2/MAP4 family of MAPs that have a similar microtubule binding region at their carboxy-terminal half. In tauopathies, such as Alzheimer's disease, tau is distributed more in the somatodendritic compartment, where it aggregates into filamentous structures, the formation of which correlates with cognitive impairments in patients. While microtubules are the dominant interaction partners of tau under physiological conditions, tau has many additional interaction partners that can contribute to its physiological and pathological role. In particular, the amino-terminal non-microtubule binding domain (N-terminal projection region, NTR) of tau interacts with many partners that are involved in membrane organization. The NTR contains intrinsically disordered regions (IDRs) that show a strong evolutionary increase in the disorder and may have been the basis for the development of new, tau-specific interactions. In this review we discuss the functional organization of the tau protein and the special features of the tau non-microtubule binding region also in the connection with the results of Tau KO models. We consider possible physiological and pathological functions of tau's non-microtubule interactions, which could indicate that interactions mediated by tau's NTR and regulated by far-reaching functional interactions of the PRR and the extreme C-terminus of tau contribute to the pathological processes.
Keywords: Alzheimer's disease; membranes; microtubule-associated protein; tau; tauopathy.
Copyright © 2020 Brandt, Trushina and Bakota.
Figures


Similar articles
-
The Evolution of Tau Phosphorylation and Interactions.Front Aging Neurosci. 2019 Sep 18;11:256. doi: 10.3389/fnagi.2019.00256. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31619983 Free PMC article.
-
Annexins A2 and A6 interact with the extreme N terminus of tau and thereby contribute to tau's axonal localization.J Biol Chem. 2018 May 25;293(21):8065-8076. doi: 10.1074/jbc.RA117.000490. Epub 2018 Apr 10. J Biol Chem. 2018. PMID: 29636414 Free PMC article.
-
Independent tubulin binding and polymerization by the proline-rich region of Tau is regulated by Tau's N-terminal domain.J Biol Chem. 2019 Dec 13;294(50):19381-19394. doi: 10.1074/jbc.RA119.010172. Epub 2019 Nov 7. J Biol Chem. 2019. PMID: 31699899 Free PMC article.
-
Systemic and network functions of the microtubule-associated protein tau: Implications for tau-based therapies.Mol Cell Neurosci. 2017 Oct;84:132-141. doi: 10.1016/j.mcn.2017.03.003. Epub 2017 Mar 17. Mol Cell Neurosci. 2017. PMID: 28318914 Review.
-
The MAP2/Tau family of microtubule-associated proteins.Genome Biol. 2005;6(1):204. doi: 10.1186/gb-2004-6-1-204. Epub 2004 Dec 23. Genome Biol. 2005. PMID: 15642108 Free PMC article. Review.
Cited by
-
Space-occupying brain lesions, trauma-related tau astrogliopathy, and ARTAG: a report of two cases and a literature review.Acta Neuropathol Commun. 2021 Mar 23;9(1):49. doi: 10.1186/s40478-021-01152-3. Acta Neuropathol Commun. 2021. PMID: 33757579 Free PMC article. Review.
-
What's in a Gene? The Outstanding Diversity of MAPT.Cells. 2022 Mar 1;11(5):840. doi: 10.3390/cells11050840. Cells. 2022. PMID: 35269461 Free PMC article. Review.
-
The Structure Biology of Tau and Clue for Aggregation Inhibitor Design.Protein J. 2021 Oct;40(5):656-668. doi: 10.1007/s10930-021-10017-6. Epub 2021 Aug 17. Protein J. 2021. PMID: 34401998 Review.
-
Loss of TMEM106B exacerbates Tau pathology and neurodegeneration in PS19 mice.Acta Neuropathol. 2024 Mar 25;147(1):62. doi: 10.1007/s00401-024-02702-4. Acta Neuropathol. 2024. PMID: 38526799
-
Deregulated Transcription and Proteostasis in Adult mapt Knockout Mouse.Int J Mol Sci. 2023 Mar 31;24(7):6559. doi: 10.3390/ijms24076559. Int J Mol Sci. 2023. PMID: 37047532 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

