SLX4IP Promotes Telomere Maintenance in Androgen Receptor-Independent Castration-Resistant Prostate Cancer through ALT-like Telomeric PML Localization
- PMID: 33188147
- PMCID: PMC8086381
- DOI: 10.1158/1541-7786.MCR-20-0314
SLX4IP Promotes Telomere Maintenance in Androgen Receptor-Independent Castration-Resistant Prostate Cancer through ALT-like Telomeric PML Localization
Abstract
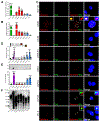
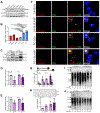
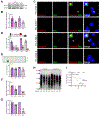
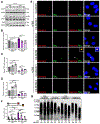
In advanced prostate cancer, resistance to androgen deprivation therapy is achieved through numerous mechanisms, including loss of the androgen receptor (AR) allowing for AR-independent growth. Therapeutic options are limited for AR-independent castration-resistant prostate cancer (CRPC), and defining mechanisms critical for survival is of utmost importance for targeting this lethal disease. Our studies focus on identifying telomere maintenance mechanism (TMM) hallmarks adopted by CRPC to promote survival. TMMs are responsible for telomere elongation to instill replicative immortality and prevent senescence, with the two TMM pathways available being telomerase and alternative lengthening of telomeres (ALT). Here, we show that AR-independent CRPC demonstrates an atypical ALT-like phenotype with variable telomerase expression and activity, whereas AR-dependent models lack discernible ALT hallmarks. In addition, AR-independent CRPC cells exhibited elevated levels of SLX4IP, a protein implicated in promoting ALT. SLX4IP overexpression in AR-dependent C4-2B cells promoted an ALT-like phenotype and telomere maintenance. SLX4IP knockdown in AR-independent DU145 and PC-3 cells led to ALT-like hallmark reduction, telomere shortening, and induction of senescence. In PC-3 xenografts, this effect translated to reduced tumor volume. Using an in vitro model of AR-independent progression, loss of AR in AR-dependent C4-2B cells promoted an atypical ALT-like phenotype in an SLX4IP-dependent manner. Insufficient SLX4IP expression diminished ALT-like hallmarks and resulted in accelerated telomere loss and senescence. IMPLICATIONS: This study demonstrates a unique reliance of AR-independent CRPC on SLX4IP-mediated ALT-like hallmarks and loss of these hallmarks induces telomere shortening and senescence, thereby impairing replicative immortality.
©2020 American Association for Cancer Research.
Figures



Similar articles
-
SLX4IP N-terminus dictates telomeric localization in ALT-like castration-resistant prostate cancer cell lines.Prostate. 2021 Nov;81(15):1235-1251. doi: 10.1002/pros.24225. Epub 2021 Sep 7. Prostate. 2021. PMID: 34492133 Free PMC article.
-
SLX4IP Antagonizes Promiscuous BLM Activity during ALT Maintenance.Mol Cell. 2019 Oct 3;76(1):27-43.e11. doi: 10.1016/j.molcel.2019.07.010. Epub 2019 Aug 22. Mol Cell. 2019. PMID: 31447390 Free PMC article.
-
SLX4IP and telomere dynamics dictate breast cancer metastasis and therapeutic responsiveness.Life Sci Alliance. 2020 Feb 18;3(4):e201900427. doi: 10.26508/lsa.201900427. Print 2020 Apr. Life Sci Alliance. 2020. PMID: 32071280 Free PMC article.
-
Androgen receptor-dependent and -independent mechanisms driving prostate cancer progression: Opportunities for therapeutic targeting from multiple angles.Oncotarget. 2017 Jan 10;8(2):3724-3745. doi: 10.18632/oncotarget.12554. Oncotarget. 2017. PMID: 27741508 Free PMC article. Review.
-
Androgen receptor: what we know and what we expect in castration-resistant prostate cancer.Int Urol Nephrol. 2018 Oct;50(10):1753-1764. doi: 10.1007/s11255-018-1964-0. Epub 2018 Aug 20. Int Urol Nephrol. 2018. PMID: 30128923 Review.
Cited by
-
TRIMming Down Hormone-Driven Cancers: The Biological Impact of TRIM Proteins on Tumor Development, Progression and Prognostication.Cells. 2021 Jun 16;10(6):1517. doi: 10.3390/cells10061517. Cells. 2021. PMID: 34208621 Free PMC article. Review.
-
SLX4IP promotes RAP1 SUMOylation by PIAS1 to coordinate telomere maintenance through NF-κB and Notch signaling.Sci Signal. 2021 Jun 29;14(689):eabe9613. doi: 10.1126/scisignal.abe9613. Sci Signal. 2021. PMID: 34187905 Free PMC article.
-
SLX4IP N-terminus dictates telomeric localization in ALT-like castration-resistant prostate cancer cell lines.Prostate. 2021 Nov;81(15):1235-1251. doi: 10.1002/pros.24225. Epub 2021 Sep 7. Prostate. 2021. PMID: 34492133 Free PMC article.
References
-
- Salonen AJ, Taari K, Ala-Opas M, Viitanen J, Lundstedt S, Tammela TLJ. The FinnProstate study VII: intermittent versus continuous androgen deprivation in patients with advanced prostate cancer. J Urol 2012;187:2074–81. - PubMed
-
- Mohler JL, Antonarakis ES, Armstrong AJ, D’Amico AV, Davis BJ, Dorff T, et al. Prostate cancer, version 2.2019. JNCCN J Natl Compr Cancer Netw 2019;17:479–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

