Intracellular β1-Adrenergic Receptors and Organic Cation Transporter 3 Mediate Phospholamban Phosphorylation to Enhance Cardiac Contractility
- PMID: 33183171
- PMCID: PMC7856104
- DOI: 10.1161/CIRCRESAHA.120.317452
Intracellular β1-Adrenergic Receptors and Organic Cation Transporter 3 Mediate Phospholamban Phosphorylation to Enhance Cardiac Contractility
Abstract
Rationale: β1ARs (β1-adrenoceptors) exist at intracellular membranes and OCT3 (organic cation transporter 3) mediates norepinephrine entry into cardiomyocytes. However, the functional role of intracellular β1AR in cardiac contractility remains to be elucidated.
Objective: Test localization and function of intracellular β1AR on cardiac contractility.
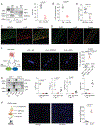
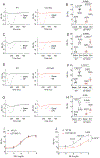
Methods and results: Membrane fractionation, super-resolution imaging, proximity ligation, coimmunoprecipitation, and single-molecule pull-down demonstrated a pool of β1ARs in mouse hearts that were associated with sarco/endoplasmic reticulum Ca2+-ATPase at the sarcoplasmic reticulum (SR). Local PKA (protein kinase A) activation was measured using a PKA biosensor targeted at either the plasma membrane (PM) or SR. Compared with wild-type, myocytes lacking OCT3 (OCT3-KO [OCT3 knockout]) responded identically to the membrane-permeant βAR agonist isoproterenol in PKA activation at both PM and SR. The same was true at the PM for membrane-impermeant norepinephrine, but the SR response to norepinephrine was suppressed in OCT3-KO myocytes. This differential effect was recapitulated in phosphorylation of the SR-pump regulator phospholamban. Similarly, OCT3-KO selectively suppressed calcium transients and contraction responses to norepinephrine but not isoproterenol. Furthermore, sotalol, a membrane-impermeant βAR-blocker, suppressed isoproterenol-induced PKA activation at the PM but permitted PKA activation at the SR, phospholamban phosphorylation, and contractility. Moreover, pretreatment with sotalol in OCT3-KO myocytes prevented norepinephrine-induced PKA activation at both PM and the SR and contractility.
Conclusions: Functional β1ARs exists at the SR and is critical for PKA-mediated phosphorylation of phospholamban and cardiac contractility upon catecholamine stimulation. Activation of these intracellular β1ARs requires catecholamine transport via OCT3.
Keywords: catecholamine; intracellular membrane; norepinephrine; phospholamban; phosphorylation.
Figures





Similar articles
-
Monoamine oxidase A and organic cation transporter 3 coordinate intracellular β1AR signaling to calibrate cardiac contractile function.Basic Res Cardiol. 2022 Jul 17;117(1):37. doi: 10.1007/s00395-022-00944-5. Basic Res Cardiol. 2022. PMID: 35842861 Free PMC article.
-
Dynamic protein kinase a activities induced by beta-adrenoceptors dictate signaling propagation for substrate phosphorylation and myocyte contraction.Circ Res. 2009 Mar 27;104(6):770-9. doi: 10.1161/CIRCRESAHA.108.187880. Epub 2009 Feb 12. Circ Res. 2009. PMID: 19213958 Free PMC article.
-
Heterologous desensitization of cardiac β-adrenergic signal via hormone-induced βAR/arrestin/PDE4 complexes.Cardiovasc Res. 2017 May 1;113(6):656-670. doi: 10.1093/cvr/cvx036. Cardiovasc Res. 2017. PMID: 28339772 Free PMC article.
-
A long lasting β1 adrenergic receptor stimulation of cAMP/protein kinase A (PKA) signal in cardiac myocytes.J Biol Chem. 2014 May 23;289(21):14771-81. doi: 10.1074/jbc.M113.542589. Epub 2014 Apr 8. J Biol Chem. 2014. PMID: 24713698 Free PMC article.
-
Phospholamban interactome in cardiac contractility and survival: A new vision of an old friend.J Mol Cell Cardiol. 2014 Dec;77:160-7. doi: 10.1016/j.yjmcc.2014.10.005. Epub 2014 Oct 18. J Mol Cell Cardiol. 2014. PMID: 25451386 Free PMC article. Review.
Cited by
-
Beta-blockers in cardiac arrhythmias-Clinical pharmacologist's point of view.Front Pharmacol. 2023 Jan 9;13:1043714. doi: 10.3389/fphar.2022.1043714. eCollection 2022. Front Pharmacol. 2023. PMID: 36699057 Free PMC article. Review.
-
Differential Downregulation of β1-Adrenergic Receptor Signaling in the Heart.J Am Heart Assoc. 2024 Jun 18;13(12):e033733. doi: 10.1161/JAHA.123.033733. Epub 2024 Jun 11. J Am Heart Assoc. 2024. PMID: 38860414 Free PMC article.
-
Carvedilol Activates a Myofilament Signaling Circuitry to Restore Cardiac Contractility in Heart Failure.JACC Basic Transl Sci. 2024 May 29;9(8):982-1001. doi: 10.1016/j.jacbts.2024.03.007. eCollection 2024 Aug. JACC Basic Transl Sci. 2024. PMID: 39297139 Free PMC article.
-
Targeting OCT3 attenuates doxorubicin-induced cardiac injury.Proc Natl Acad Sci U S A. 2021 Feb 2;118(5):e2020168118. doi: 10.1073/pnas.2020168118. Proc Natl Acad Sci U S A. 2021. PMID: 33495337 Free PMC article.
-
Rescue of Misfolded Organic Cation Transporter 3 Variants.Cells. 2022 Dec 22;12(1):39. doi: 10.3390/cells12010039. Cells. 2022. PMID: 36611832 Free PMC article.
References
-
- Boivin B, Lavoie C, Vaniotis G, Baragli A, Villeneuve LR, Ethier N, Trieu P, Allen BG and Hebert TE. Functional beta-adrenergic receptor signalling on nuclear membranes in adult rat and mouse ventricular cardiomyocytes. Cardiovasc Res. 2006;71:69–78. - PubMed
-
- Ibarra C, Vicencio JM, Estrada M, Lin Y, Rocco P, Rebellato P, Munoz JP, Garcia-Prieto J, Quest AF, Chiong M, Davidson SM, Bulatovic I, Grinnemo KH, Larsson O, Szabadkai G, Uhlen P, Jaimovich E and Lavandero S. Local control of nuclear calcium signaling in cardiac myocytes by perinuclear microdomains of sarcolemmal insulin-like growth factor 1 receptors. Circ Res. 2013;112:236–45. - PubMed
-
- Benard G, Massa F, Puente N, Lourenco J, Bellocchio L, Soria-Gomez E, Matias I, Delamarre A, Metna-Laurent M, Cannich A, Hebert-Chatelain E, Mulle C, Ortega-Gutierrez S, Martin-Fontecha M, Klugmann M, Guggenhuber S, Lutz B, Gertsch J, Chaouloff F, Lopez-Rodriguez ML, Grandes P, Rossignol R and Marsicano G. Mitochondrial CB(1) receptors regulate neuronal energy metabolism. Nature neuroscience. 2012;15:558–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

