Lymphotropic Viruses: Chronic Inflammation and Induction of Cancers
- PMID: 33182552
- PMCID: PMC7697807
- DOI: 10.3390/biology9110390
Lymphotropic Viruses: Chronic Inflammation and Induction of Cancers
Abstract
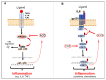
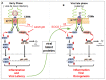
Inflammation induced by transcription factors, including Signal Transducers and Activators of Transcription (STATs) and NF-κB, in response to microbial pathogenic infections and ligand dependent receptors stimulation are critical for controlling infections. However, uncontrolled inflammation induced by these transcription factors could lead to immune dysfunction, persistent infection, inflammatory related diseases and the development of cancers. Although the induction of innate immunity and inflammation in response to viral infection is important to control virus replication, its effects can be modulated by lymphotropic viruses including human T-cell leukemia virus type 1 (HTLV-1), Κaposi's sarcoma herpesvirus (KSHV), and Epstein Barr virus (EBV) during de novo infection as well as latent infection. These lymphotropic viruses persistently activate JAK-STAT and NF-κB pathways. Long-term STAT and NF-κB activation by these viruses leads to the induction of chronic inflammation, which can support the persistence of these viruses and promote virus-mediated cancers. Here, we review how HTLV-1, KSHV and EBV hijack the function of host cell surface molecules (CSMs), which are involved in the regulation of chronic inflammation, innate and adaptive immune responses, cell death and the restoration of tissue homeostasis. Thus, better understanding of CSMs-mediated chronic activation of STATs and NF-κB pathways in lymphotropic virus-infected cells may pave the way for therapeutic intervention in malignancies caused by lymphotropic viruses.
Keywords: EBV; HTLV-1; NF-κB; STAT; cancers; cell surface molecules (CSMs); inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Regulation of Autophagy in Cells Infected With Oncogenic Human Viruses and Its Impact on Cancer Development.Front Cell Dev Biol. 2020 Feb 28;8:47. doi: 10.3389/fcell.2020.00047. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32181249 Free PMC article. Review.
-
Control of the inflammatory response mechanisms mediated by natural and induced regulatory T-cells in HCV-, HTLV-1-, and EBV-associated cancers.Mediators Inflamm. 2014;2014:564296. doi: 10.1155/2014/564296. Epub 2014 Nov 30. Mediators Inflamm. 2014. PMID: 25525301 Free PMC article. Review.
-
Multiple signal transducers and activators of transcription are induced by EBV LMP-1.Virology. 2004 May 20;323(1):141-52. doi: 10.1016/j.virol.2004.03.007. Virology. 2004. PMID: 15165826
-
The interplay between EBV and KSHV viral products and NF-κB pathway in oncogenesis.Infect Agent Cancer. 2020 Oct 15;15:62. doi: 10.1186/s13027-020-00317-4. eCollection 2020. Infect Agent Cancer. 2020. PMID: 33072180 Free PMC article. Review.
-
The Major Histocompatibility Complex Class II Transactivator CIITA Inhibits the Persistent Activation of NF-κB by the Human T Cell Lymphotropic Virus Type 1 Tax-1 Oncoprotein.J Virol. 2016 Jan 20;90(7):3708-21. doi: 10.1128/JVI.03000-15. J Virol. 2016. PMID: 26792751 Free PMC article.
Cited by
-
New Look of EBV LMP1 Signaling Landscape.Cancers (Basel). 2021 Oct 29;13(21):5451. doi: 10.3390/cancers13215451. Cancers (Basel). 2021. PMID: 34771613 Free PMC article. Review.
-
Association between the systemic inflammatory response index and mortality in patients with sarcopenia.PLoS One. 2024 Nov 18;19(11):e0312383. doi: 10.1371/journal.pone.0312383. eCollection 2024. PLoS One. 2024. PMID: 39556533 Free PMC article.
-
Potential inhibitors interacting in Neuropilin-1 to develop an adjuvant drug against COVID-19, by molecular docking.Bioorg Med Chem. 2021 Mar 1;33:116040. doi: 10.1016/j.bmc.2021.116040. Epub 2021 Jan 23. Bioorg Med Chem. 2021. PMID: 33515918 Free PMC article.
-
Human T-cell lymphotropic virus type 1 (HTLV-1) grip on T-cells: investigating the viral tapestry of activation.Infect Agent Cancer. 2024 May 11;19(1):23. doi: 10.1186/s13027-024-00584-5. Infect Agent Cancer. 2024. PMID: 38734673 Free PMC article.
-
The potential effects and mechanisms of breast inflammatory lesions on the occurrence and development of breast cancer.Front Oncol. 2022 Aug 5;12:932743. doi: 10.3389/fonc.2022.932743. eCollection 2022. Front Oncol. 2022. PMID: 35992864 Free PMC article.
References
-
- Coussens L. Session 2: Inflammation and Cancer. Toxicol. Pathol. 2004;32:732. doi: 10.1080/01926230490882402. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

