Dominance of the ON1 Genotype of RSV-A and BA9 Genotype of RSV-B in Respiratory Cases from Jeddah, Saudi Arabia
- PMID: 33182267
- PMCID: PMC7695323
- DOI: 10.3390/genes11111323
Dominance of the ON1 Genotype of RSV-A and BA9 Genotype of RSV-B in Respiratory Cases from Jeddah, Saudi Arabia
Abstract
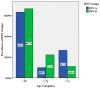
Human respiratory syncytial virus (HRSV) is a main cause of hospital admission for lower respiratory tract infection. In previous studies from Saudi Arabia, higher prevalence of the NA1 genotype in group A was observed from Riyadh and Taif. This study recruited respiratory cases from Jeddah during January to December, 2017. RSV represented 13.4% in the recruited cases with 64% of them belonging to group A and 36% to group B. All group A cases in this study were ON1 type characterized by duplication of 72 nucleotides, 24 amino acids in the C-terminal in the second hypervariable region of the G gene. In addition, for group B all of the cases were clustered under BA9, which had uniquely characterized as duplication of 60 nucleotides in the G protein. Our sequences showed similarity with earlier sequences from Saudi Arabia, Kuwait, Thailand, South Africa, Spain, the USA and Cyprus. Some amino acid substitutions in the investigated sequences would cause a change in potential O-glycosylation and N-glycosylation profiles from prototype ON1. The predominance of the ON1 and BA9 genotype of RSV-A in Jeddah compared to previous Saudi studies showing predominance of the NA1 genotype for group A. This difference in genotype prevalence could be due to fast spread of the ON1 genotype worldwide or due to the flux of travelers through Jeddah during hajj/umrah compared to Riyadh and Taif. This shift in genotype distribution requires continuous surveillance for genetic characterization of circulating respiratory infections including RSV. These findings may contribute to the understanding of RSV evolution and to the potential development of a vaccine against RSV.
Keywords: RSV-A; RSV-B; Saudi Arabia; phylogenetic analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Co-Circulation of 72bp Duplication Group A and 60bp Duplication Group B Respiratory Syncytial Virus (RSV) Strains in Riyadh, Saudi Arabia during 2014.PLoS One. 2016 Nov 11;11(11):e0166145. doi: 10.1371/journal.pone.0166145. eCollection 2016. PLoS One. 2016. PMID: 27835664 Free PMC article.
-
Evolutionary analysis of the ON1 genotype of subtype a respiratory syncytial virus in Riyadh during 2008-16.Infect Genet Evol. 2020 Apr;79:104153. doi: 10.1016/j.meegid.2019.104153. Epub 2019 Dec 24. Infect Genet Evol. 2020. PMID: 31881360
-
A multi-center study on Molecular Epidemiology of Human Respiratory Syncytial Virus from Children with Acute Lower Respiratory Tract Infections in the Mainland of China between 2015 and 2019.Virol Sin. 2021 Dec;36(6):1475-1483. doi: 10.1007/s12250-021-00430-7. Epub 2021 Aug 16. Virol Sin. 2021. PMID: 34398429 Free PMC article.
-
Respiratory syncytial virus subtype ON1/NA1/BA9 predominates in hospitalized children with lower respiratory tract infections.J Med Virol. 2017 Feb;89(2):213-221. doi: 10.1002/jmv.24619. Epub 2016 Jul 6. J Med Virol. 2017. PMID: 27358012 Free PMC article.
-
The contribution of gastrointestinal microbiota in the existence of type 2 diabetes in Saudi Arabia: Current information and perspectives.Saudi J Biol Sci. 2022 Jun;29(6):103286. doi: 10.1016/j.sjbs.2022.103286. Epub 2022 Apr 21. Saudi J Biol Sci. 2022. PMID: 35602871 Free PMC article. Review.
Cited by
-
The Modification of the Illumina® CovidSeq™ Workflow for RSV Genomic Surveillance: The Genetic Variability of RSV during the 2022-2023 Season in Northwest Spain.Int J Mol Sci. 2023 Nov 7;24(22):16055. doi: 10.3390/ijms242216055. Int J Mol Sci. 2023. PMID: 38003246 Free PMC article.
-
Comparative analysis of human respiratory syncytial virus evolutionary patterns during the COVID-19 pandemic and pre-pandemic periods.Front Microbiol. 2023 Dec 4;14:1298026. doi: 10.3389/fmicb.2023.1298026. eCollection 2023. Front Microbiol. 2023. PMID: 38111642 Free PMC article.
-
Epidemiology and genetic diversity of human respiratory syncytial virus in Belgium between 2011 and 2019.Virol J. 2024 Oct 28;21(1):270. doi: 10.1186/s12985-024-02542-4. Virol J. 2024. PMID: 39468663 Free PMC article.
-
The Scope of Respiratory Syncytial Virus Infection in a Tertiary Hospital in the Eastern Province of Saudi Arabia and the Change in Seasonal Pattern during and after the COVID-19 Pandemic.Medicina (Kaunas). 2022 Nov 10;58(11):1623. doi: 10.3390/medicina58111623. Medicina (Kaunas). 2022. PMID: 36363580 Free PMC article.
-
Epidemiology and clinical features of respiratory syncytial virus (RSV) infection in hospitalized children during the COVID-19 pandemic in Gorgan, Iran.Health Sci Rep. 2024 Jan 3;7(1):e1787. doi: 10.1002/hsr2.1787. eCollection 2024 Jan. Health Sci Rep. 2024. PMID: 38186938 Free PMC article.
References
-
- Yu X., Kou Y., Xia D., Li J., Yang X., Zhou Y., He X. Human respiratory syncytial virus in children with lower respiratory tract infections or influenza-like illness and its co-infection characteristics with viruses and atypical bacteria in Hangzhou, China. J. Clin. Virol. 2015;69:1–6. doi: 10.1016/j.jcv.2015.05.015. - DOI - PMC - PubMed
-
- Nair H., Nokes D.J., Gessner B.D., Dherani M., Madhi S.A., Singleton R.J., O’Brien K.L., Roca A., Wright P.F., Bruce N. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic reviewand meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. - DOI - PMC - PubMed
-
- Eshaghi A., Duvvuri V.R., Lai R., Nadarajah J.T., Li A., Patel S.N., Low D.E., Gubbay J.B. Genetic variability of human respiratory syncytial virus A strainscirculating in Ontario: A novel genotype with a 72 nucleotide G gene duplication. PLoS ONE. 2012;7:e32807. doi: 10.1371/journal.pone.0032807. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

