Manganese exposure induces permeability in renal glomerular endothelial cells via the Smad2/3-Snail-VE-cadherin axis
- PMID: 33178429
- PMCID: PMC7640925
- DOI: 10.1093/toxres/tfaa067
Manganese exposure induces permeability in renal glomerular endothelial cells via the Smad2/3-Snail-VE-cadherin axis
Abstract
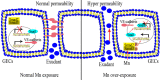
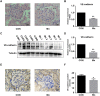
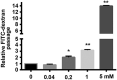
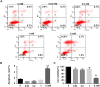
Manganese (Mn) is an essential micronutrient. However, it is well established that Mn overexposure causes nervous system diseases. In contrast, there are few reports on the effects of Mn exposure on glomerular endothelium. In the present study, the potential effects of Mn exposure on glomerular endothelium were evaluated. Sprague Dawley rats were used as a model of Mn overexposure by intraperitoneal injection of MnCl2·H2O at 25 mg/kg body weight. Mn exposure decreased expression of vascular endothelial-cadherin, a key component of adherens junctions, and increased exudate from glomeruli in Sprague Dawley rats. Human renal glomerular endothelial cells were cultured with different concentration of Mn. Exposure to 0.2 mM Mn increased permeability of human renal glomerular endothelial cell monolayers and decreased vascular endothelial-cadherin expression without inducing cytotoxicity. In addition, Mn exposure increased phosphorylation of mothers against decapentaplegic homolog 2/3 and upregulated expression of zinc finger protein SNAI1, a negative transcriptional regulator of vascular endothelial-cadherin. Our data suggest Mn exposure may contribute to development of glomerular diseases by inducing permeability of glomerular endothelium.
Keywords: VE-cadherin; adherens junction; glomerular endothelial cells; glomerular filtration barrier; manganese.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
The impact of manganese on vascular endothelium.Toxicol Res. 2024 Aug 13;40(4):501-517. doi: 10.1007/s43188-024-00260-1. eCollection 2024 Oct. Toxicol Res. 2024. PMID: 39345740 Free PMC article. Review.
-
Aberrant Activation of Notch1 Signaling in Glomerular Endothelium Induces Albuminuria.Circ Res. 2021 Mar 5;128(5):602-618. doi: 10.1161/CIRCRESAHA.120.316970. Epub 2021 Jan 13. Circ Res. 2021. PMID: 33435713
-
Activation of Vascular Endothelial Growth Factor (VEGF) Receptor 2 Mediates Endothelial Permeability Caused by Cyclic Stretch.J Biol Chem. 2016 May 6;291(19):10032-45. doi: 10.1074/jbc.M115.690487. Epub 2016 Feb 16. J Biol Chem. 2016. PMID: 26884340 Free PMC article.
-
Short-term, low-dose cadmium exposure induces hyperpermeability in human renal glomerular endothelial cells.J Appl Toxicol. 2016 Feb;36(2):257-65. doi: 10.1002/jat.3168. Epub 2015 May 25. J Appl Toxicol. 2016. PMID: 26011702
-
Dynamic Regulation of Vascular Permeability by Vascular Endothelial Cadherin-Mediated Endothelial Cell-Cell Junctions.J Nippon Med Sch. 2017;84(4):148-159. doi: 10.1272/jnms.84.148. J Nippon Med Sch. 2017. PMID: 28978894 Review.
Cited by
-
Association of Manganese Biomarker Concentrations with Blood Pressure and Kidney Parameters among Healthy Adolescents: NHANES 2013-2018.Children (Basel). 2021 Sep 25;8(10):846. doi: 10.3390/children8100846. Children (Basel). 2021. PMID: 34682111 Free PMC article.
-
The impact of manganese on vascular endothelium.Toxicol Res. 2024 Aug 13;40(4):501-517. doi: 10.1007/s43188-024-00260-1. eCollection 2024 Oct. Toxicol Res. 2024. PMID: 39345740 Free PMC article. Review.
-
MiR-195 inhibits the ubiquitination and degradation of YY1 by Smurf2, and induces EMT and cell permeability of retinal pigment epithelial cells.Cell Death Dis. 2021 Jul 15;12(7):708. doi: 10.1038/s41419-021-03956-6. Cell Death Dis. 2021. PMID: 34267179 Free PMC article.
-
STAT6 Upregulates NRP1 Expression in Endothelial Cells and Promotes Angiogenesis.Front Oncol. 2022 May 5;12:823377. doi: 10.3389/fonc.2022.823377. eCollection 2022. Front Oncol. 2022. PMID: 35600336 Free PMC article.
-
Dihydroartemisinin protects blood-brain barrier permeability during sepsis by inhibiting the transcription factor SNAI1.Clin Exp Pharmacol Physiol. 2022 Sep;49(9):979-987. doi: 10.1111/1440-1681.13683. Epub 2022 Jun 24. Clin Exp Pharmacol Physiol. 2022. PMID: 35651290 Free PMC article.
References
-
- Chen P, Bornhorst J, Aschner M. Manganese metabolism in humans. Front Biosci (Landmark Ed) 2018;23:1655–79. - PubMed
-
- Liu K, Jing MJ, Liu C et al. . Effect of trehalose on manganese-induced mitochondrial dysfunction and neuronal cell damage in mice. Basic Clin Pharmacol Toxicol 2019;125:536–47. - PubMed
-
- Richter Schmitz CR, Eichwald T, Branco Flores MV et al. . Sex differences in subacute manganese intoxication: oxidative parameters and metal deposition in peripheral organs of adult Wistar rats. Regul Toxicol Pharmacol 2019;104:98–107. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

