Screening of a Panel of Low Molecular Weight Compounds That Inhibit Synovial Fibroblast Invasion in Rheumatoid Arthritis
- PMID: 33177160
- PMCID: PMC7718771
- DOI: 10.4049/jimmunol.1901429
Screening of a Panel of Low Molecular Weight Compounds That Inhibit Synovial Fibroblast Invasion in Rheumatoid Arthritis
Abstract
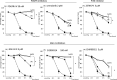
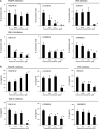
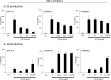
Increased invasion of synovial fibroblasts and their involvement in cartilage damage are characteristic phenotypes of rheumatoid arthritis (RA). To identify low molecular weight compounds that suppress synovial fibroblast invasion, a panel of inhibitors (n = 330) was initially screened using a real-time cell analysis system for human synovial fibroblasts that were enzymatically isolated from surgical samples of RA patients. To evaluate the effects of the inhibitors identified in the screen, synovial fibroblast migration was measured using a wound-healing assay, and phosphorylation of intracellular signaling molecules was determined by immunoblots. Several candidate inhibitors were identified in the screen, including inhibitors against platelet-derived growth factor receptor (PDGFR), Akt, PI3K, and glycogen kinase synthetase 3 (GSK-3). These inhibitors strongly suppressed synovial fibroblast migration after 72 h and downregulated phosphorylation of Akt (Ser473) at 48 h. When the inhibitors were removed from the culture conditions, both migration and phosphorylated Akt (Ser473) levels were restored. Furthermore, all the categories of inhibitors except for PDGFR inhibitor IV decreased cell proliferation as well as IL-6 production in synovial fibroblasts. Interestingly, GSK-3 inhibitors increased anti-inflammatory cytokine IL-10 production but suppressed IL-23 production from LPS-primed macrophages obtained from healthy donors. In conclusion, blocking PDGFR, PI3K, or GSK-3 could have therapeutic value as an RA treatment that targets the invasion/migration of synovial fibroblasts.
Copyright © 2020 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures






Similar articles
-
A Novel Phytochemical, DIM, Inhibits Proliferation, Migration, Invasion and TNF-α Induced Inflammatory Cytokine Production of Synovial Fibroblasts From Rheumatoid Arthritis Patients by Targeting MAPK and AKT/mTOR Signal Pathway.Front Immunol. 2019 Jul 23;10:1620. doi: 10.3389/fimmu.2019.01620. eCollection 2019. Front Immunol. 2019. PMID: 31396207 Free PMC article.
-
Endogenous MMP-9 and not MMP-2 promotes rheumatoid synovial fibroblast survival, inflammation and cartilage degradation.Rheumatology (Oxford). 2014 Dec;53(12):2270-9. doi: 10.1093/rheumatology/keu254. Epub 2014 Jun 29. Rheumatology (Oxford). 2014. PMID: 24982240
-
Focal adhesion kinase is required for synovial fibroblast invasion, but not murine inflammatory arthritis.Arthritis Res Ther. 2014 Oct 4;16(5):464. doi: 10.1186/s13075-014-0464-6. Arthritis Res Ther. 2014. PMID: 25280866 Free PMC article.
-
[Rheumatoid arthritis: new developments in the pathogenesis with special reference to synovial fibroblasts].Z Rheumatol. 2001 Oct;60(5):309-18. doi: 10.1007/s003930170030. Z Rheumatol. 2001. PMID: 11759230 Review. German.
-
The Role of Extracellular Nucleic Acids in Rheumatoid Arthritis.Curr Pharm Biotechnol. 2018;19(15):1182-1188. doi: 10.2174/1389201020666190102150216. Curr Pharm Biotechnol. 2018. PMID: 30605055 Review.
Cited by
-
Synovial fibroblasts as potential drug targets in rheumatoid arthritis, where do we stand and where shall we go?Ann Rheum Dis. 2022 Jun 17;81(8):1055-64. doi: 10.1136/annrheumdis-2021-222021. Online ahead of print. Ann Rheum Dis. 2022. PMID: 35715191 Free PMC article. Review.
-
Myricitrin inhibits fibroblast-like synoviocyte-mediated rheumatoid synovial inflammation and joint destruction by targeting AIM2.Front Pharmacol. 2022 Aug 31;13:905376. doi: 10.3389/fphar.2022.905376. eCollection 2022. Front Pharmacol. 2022. PMID: 36120327 Free PMC article.
-
Advances of the small molecule drugs regulating fibroblast-like synovial proliferation for rheumatoid arthritis.Front Pharmacol. 2023 Jul 21;14:1230293. doi: 10.3389/fphar.2023.1230293. eCollection 2023. Front Pharmacol. 2023. PMID: 37547337 Free PMC article. Review.
References
-
- Cooper N. J. 2000. Economic burden of rheumatoid arthritis: a systematic review. Rheumatology (Oxford) 39: 28–33. - PubMed
-
- Lafyatis R., Remmers E. F., Roberts A. B., Yocum D. E., Sporn M. B., Wilder R. L. 1989. Anchorage-independent growth of synoviocytes from arthritic and normal joints. Stimulation by exogenous platelet-derived growth factor and inhibition by transforming growth factor-beta and retinoids. J. Clin. Invest. 83: 1267–1276. - PMC - PubMed
-
- Harigai M., Hara M., Kawamoto M., Kawaguchi Y., Sugiura T., Tanaka M., Nakagawa M., Ichida H., Takagi K., Higami-Ohsako S., et al. 2004. Amplification of the synovial inflammatory response through activation of mitogen-activated protein kinases and nuclear factor kappaB using ligation of CD40 on CD14+ synovial cells from patients with rheumatoid arthritis. Arthritis Rheum. 50: 2167–2177. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

