Pancreatic β-Cell-Specific Deletion of VPS41 Causes Diabetes Due to Defects in Insulin Secretion
- PMID: 33168621
- PMCID: PMC7881869
- DOI: 10.2337/db20-0454
Pancreatic β-Cell-Specific Deletion of VPS41 Causes Diabetes Due to Defects in Insulin Secretion
Abstract
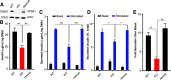
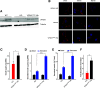
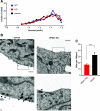
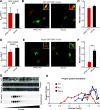
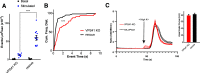
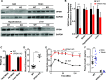
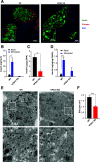
Insulin secretory granules (SGs) mediate the regulated secretion of insulin, which is essential for glucose homeostasis. The basic machinery responsible for this regulated exocytosis consists of specific proteins present both at the plasma membrane and on insulin SGs. The protein composition of insulin SGs thus dictates their release properties, yet the mechanisms controlling insulin SG formation, which determine this molecular composition, remain poorly understood. VPS41, a component of the endolysosomal tethering homotypic fusion and vacuole protein sorting (HOPS) complex, was recently identified as a cytosolic factor involved in the formation of neuroendocrine and neuronal granules. We now find that VPS41 is required for insulin SG biogenesis and regulated insulin secretion. Loss of VPS41 in pancreatic β-cells leads to a reduction in insulin SG number, changes in their transmembrane protein composition, and defects in granule-regulated exocytosis. Exploring a human point mutation, identified in patients with neurological but no endocrine defects, we show that the effect on SG formation is independent of HOPS complex formation. Finally, we report that mice with a deletion of VPS41 specifically in β-cells develop diabetes due to severe depletion of insulin SG content and a defect in insulin secretion. In sum, our data demonstrate that VPS41 contributes to glucose homeostasis and metabolism.
© 2020 by the American Diabetes Association.
Figures
Similar articles
-
A fluorescent timer reporter enables sorting of insulin secretory granules by age.J Biol Chem. 2020 Jul 3;295(27):8901-8911. doi: 10.1074/jbc.RA120.012432. Epub 2020 Apr 27. J Biol Chem. 2020. PMID: 32341128 Free PMC article.
-
Munc18b is a major mediator of insulin exocytosis in rat pancreatic β-cells.Diabetes. 2013 Jul;62(7):2416-28. doi: 10.2337/db12-1380. Epub 2013 Feb 19. Diabetes. 2013. PMID: 23423569 Free PMC article.
-
Recent new insights into the role of SNARE and associated proteins in insulin granule exocytosis.Diabetes Obes Metab. 2017 Sep;19 Suppl 1:115-123. doi: 10.1111/dom.13001. Diabetes Obes Metab. 2017. PMID: 28880475 Review.
-
Self-assembly of VPS41 promotes sorting required for biogenesis of the regulated secretory pathway.Dev Cell. 2013 Nov 25;27(4):425-37. doi: 10.1016/j.devcel.2013.10.007. Epub 2013 Nov 7. Dev Cell. 2013. PMID: 24210660 Free PMC article.
-
Insulin granule biogenesis and exocytosis.Cell Mol Life Sci. 2021 Mar;78(5):1957-1970. doi: 10.1007/s00018-020-03688-4. Epub 2020 Nov 4. Cell Mol Life Sci. 2021. PMID: 33146746 Free PMC article. Review.
Cited by
-
Endocrine secretory granule production is caused by a lack of REST and intragranular secretory content and accelerated by PROX1.J Mol Histol. 2022 Apr;53(2):437-448. doi: 10.1007/s10735-021-10055-5. Epub 2022 Jan 30. J Mol Histol. 2022. PMID: 35094211 Free PMC article.
-
Sustained hyperglycemia specifically targets translation of mRNAs for insulin secretion.J Clin Invest. 2023 Nov 30;134(3):e173280. doi: 10.1172/JCI173280. J Clin Invest. 2023. PMID: 38032734 Free PMC article.
-
Mutations in CmVPS41 controlling resistance to cucumber mosaic virus display specific subcellular localization.Plant Physiol. 2023 Mar 17;191(3):1596-1611. doi: 10.1093/plphys/kiac583. Plant Physiol. 2023. PMID: 36527697 Free PMC article.
-
Genetics and diet shape the relationship between islet function and whole body metabolism.Am J Physiol Endocrinol Metab. 2024 May 1;326(5):E663-E672. doi: 10.1152/ajpendo.00060.2024. Epub 2024 Apr 3. Am J Physiol Endocrinol Metab. 2024. PMID: 38568150 Free PMC article.
-
Emerging approaches for the development of artificial islets.Smart Med. 2024 Mar 7;3(2):e20230042. doi: 10.1002/SMMD.20230042. eCollection 2024 Jun. Smart Med. 2024. PMID: 39188698 Free PMC article. Review.
References
-
- Borgonovo B, Ouwendijk J, Solimena M. Biogenesis of secretory granules. Curr Opin Cell Biol 2006;18:365–370 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

