Inhibition of TGFβ1 and TGFβ3 promotes hematopoiesis in Fanconi anemia
- PMID: 33166613
- PMCID: PMC8686188
- DOI: 10.1016/j.exphem.2020.11.002
Inhibition of TGFβ1 and TGFβ3 promotes hematopoiesis in Fanconi anemia
Abstract
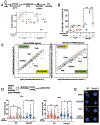
Fanconi anemia (FA) is a chromosome instability syndrome with congenital abnormalities, cancer predisposition and bone marrow failure (BMF). Although hematopoietic stem and progenitor cell (HSPC) transplantation is the recommended therapy, new therapies are needed for FA patients without suitable donors. BMF in FA is caused, at least in part, by a hyperactive growth-suppressive transforming growth factor β (TGFβ) pathway, regulated by the TGFβ1, TGFβ2, and TGFβ3 ligands. Accordingly, the TGFβ pathway is an attractive therapeutic target for FA. While inhibition of TGFβ1 and TGFβ3 promotes blood cell expansion, inhibition of TGFβ2 is known to suppress hematopoiesis. Here, we report the effects of AVID200, a potent TGFβ1- and TGFβ3-specific inhibitor, on FA hematopoiesis. AVID200 promoted the survival of murine FA HSPCs in vitro. AVID200 also promoted in vitro the survival of human HSPCs from patients with FA, with the strongest effect in patients progressing to severe aplastic anemia or myelodysplastic syndrome (MDS). Previous studies have indicated that the toxic upregulation of the nonhomologous end-joining (NHEJ) pathway accounts, at least in part, for the poor growth of FA HSPCs. AVID200 downregulated the expression of NHEJ-related genes and reduced DNA damage in primary FA HSPC in vitro and in in vivo models. Collectively, AVID200 exhibits activity in FA mouse and human preclinical models. AVID200 may therefore provide a therapeutic approach to improving BMF in FA.
Copyright © 2020 ISEH -- Society for Hematology and Stem Cells. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest disclosure ADD is a consultant/advisory board member for Lilly Oncology, Merck-EMD Serono, Intellia Therapeutics, Sierra Oncology, Cyteir Therapeutics, Third Rock Ventures, AstraZeneca, Ideaya Inc., Cedilla Therapeutics Inc.; is a stockholder in Ideaya Inc., Cedilla Therapeutics Inc., and Cyteir; and reports receiving commercial research grants from Lilly Oncology and Merck-EMD Serono. Other authors declare no conflicts of interest.
Figures
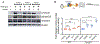


Similar articles
-
TGF-β1 protein trap AVID200 beneficially affects hematopoiesis and bone marrow fibrosis in myelofibrosis.JCI Insight. 2021 Sep 22;6(18):e145651. doi: 10.1172/jci.insight.145651. JCI Insight. 2021. PMID: 34383713 Free PMC article.
-
TGF-β Inhibition Rescues Hematopoietic Stem Cell Defects and Bone Marrow Failure in Fanconi Anemia.Cell Stem Cell. 2016 May 5;18(5):668-81. doi: 10.1016/j.stem.2016.03.002. Epub 2016 Mar 24. Cell Stem Cell. 2016. PMID: 27053300 Free PMC article.
-
Bone marrow failure in Fanconi anemia is triggered by an exacerbated p53/p21 DNA damage response that impairs hematopoietic stem and progenitor cells.Cell Stem Cell. 2012 Jul 6;11(1):36-49. doi: 10.1016/j.stem.2012.05.013. Epub 2012 Jun 7. Cell Stem Cell. 2012. PMID: 22683204 Free PMC article.
-
Current knowledge on the pathophysiology of Fanconi anemia: from genes to phenotypes.Int J Hematol. 2001 Jul;74(1):33-41. doi: 10.1007/BF02982547. Int J Hematol. 2001. PMID: 11530803 Review.
-
Oxidative stress in Fanconi anemia hematopoiesis and disease progression.Antioxid Redox Signal. 2008 Nov;10(11):1909-21. doi: 10.1089/ars.2008.2129. Antioxid Redox Signal. 2008. PMID: 18627348 Free PMC article. Review.
Cited by
-
Chemical Carcinogen (Dimethyl-benzanthracene) Induced Transplantable Cancer in Fanconi Anemia (Fanca-/-) Mice.In Vivo. 2023 Nov-Dec;37(6):2421-2432. doi: 10.21873/invivo.13347. In Vivo. 2023. PMID: 37905617 Free PMC article.
-
Isolation of human and murine hematopoietic stem cells for DNA damage and DNA repair assays.STAR Protoc. 2021 Sep 27;2(4):100846. doi: 10.1016/j.xpro.2021.100846. eCollection 2021 Dec 17. STAR Protoc. 2021. PMID: 34622219 Free PMC article.
-
TGF-beta signal transduction: biology, function and therapy for diseases.Mol Biomed. 2022 Dec 19;3(1):45. doi: 10.1186/s43556-022-00109-9. Mol Biomed. 2022. PMID: 36534225 Free PMC article. Review.
-
Genotoxic aldehyde stress prematurely ages hematopoietic stem cells in a p53-driven manner.Mol Cell. 2023 Jul 20;83(14):2417-2433.e7. doi: 10.1016/j.molcel.2023.05.035. Epub 2023 Jun 21. Mol Cell. 2023. PMID: 37348497 Free PMC article.
-
TGFβ pathway is required for viable gestation of Fanconi anemia embryos.PLoS Genet. 2022 Nov 28;18(11):e1010459. doi: 10.1371/journal.pgen.1010459. eCollection 2022 Nov. PLoS Genet. 2022. PMID: 36441774 Free PMC article.
References
-
- Ceccaldi R, Sarangi P, and D’Andrea AD, The Fanconi anaemia pathway: new players and new functions. Nat Rev Mol Cell Biol, 2016. 17(6): p. 337–49. - PubMed
-
- Tischkowitz M. and Dokal I, Fanconi anaemia and leukaemia–clinical and molecular aspects. Br. J. Haematol, 2004. 126(2): p. 176–191. - PubMed
-
- Garaycoechea JI and Patel KJ, Why does the bone marrow fail in Fanconi anemia? Blood, 2014. 123(1): p. 26–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

