Recommendations for sample pooling on the Cepheid GeneXpert® system using the Cepheid Xpert® Xpress SARS-CoV-2 assay
- PMID: 33166373
- PMCID: PMC7652318
- DOI: 10.1371/journal.pone.0241959
Recommendations for sample pooling on the Cepheid GeneXpert® system using the Cepheid Xpert® Xpress SARS-CoV-2 assay
Abstract
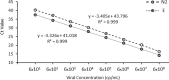
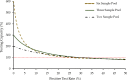
The coronavirus disease 2019 (Covid-19) pandemic, caused by SARS-CoV-2, has resulted in a global testing supply shortage. In response, pooled testing has emerged as a promising strategy that can immediately increase testing capacity. In pooled sample testing, multiple samples are combined (or pooled) together and tested as a single unit. If the pool is positive, the individual samples can then be individually tested to identify the positive case(s). Here, we provide support for the adoption of sample pooling with the point-of-care Cepheid Xpert® Xpress SARS-CoV-2 molecular assay. Corroborating previous findings, the limit of detection of this assay was comparable to laboratory-developed reverse-transcription quantitative PCR SARS-CoV-2 tests, with observed detection below 100 copies/mL. The Xpert® Xpress assay detected SARS-CoV-2 after samples with minimum viral loads of 461 copies/mL were pooled in groups of six. Based on these data, we recommend the adoption of pooled testing with the Xpert® Xpress SARS-CoV-2 assay where warranted based on public health needs. The suggested number of samples per pool, or the pooling depth, is unique for each point-of-care testing site and can be determined by the positive test rates. To statistically determine appropriate pooling depth, we have calculated the pooling efficiency for numerous combinations of pool sizes and test rates. This information is included as a supplemental dataset that we encourage public health authorities to use as a guide to make recommendations that will maximize testing capacity and resource conservation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Multi-center evaluation of cepheid xpert® xpress SARS-CoV-2 point-of-care test during the SARS-CoV-2 pandemic.J Clin Virol. 2020 Jul;128:104426. doi: 10.1016/j.jcv.2020.104426. Epub 2020 May 11. J Clin Virol. 2020. PMID: 32417674 Free PMC article.
-
Comparison of a Point-of-Care Assay and a High-Complexity Assay for Detection of SARS-CoV-2 RNA.J Appl Lab Med. 2020 Nov 1;5(6):1307-1312. doi: 10.1093/jalm/jfaa135. J Appl Lab Med. 2020. PMID: 32761092 Free PMC article.
-
Performance of Abbott ID Now COVID-19 Rapid Nucleic Acid Amplification Test Using Nasopharyngeal Swabs Transported in Viral Transport Media and Dry Nasal Swabs in a New York City Academic Institution.J Clin Microbiol. 2020 Jul 23;58(8):e01136-20. doi: 10.1128/JCM.01136-20. Print 2020 Jul 23. J Clin Microbiol. 2020. PMID: 32471894 Free PMC article.
-
Molecular and Serological Assays for SARS-CoV-2: Insights from Genome and Clinical Characteristics.Clin Chem. 2020 Aug 1;66(8):1030-1046. doi: 10.1093/clinchem/hvaa122. Clin Chem. 2020. PMID: 32437513 Free PMC article. Review.
-
Laboratory diagnosis of SARS-CoV-2 - A review of current methods.J Infect Public Health. 2020 Jul;13(7):901-905. doi: 10.1016/j.jiph.2020.06.005. Epub 2020 Jun 7. J Infect Public Health. 2020. PMID: 32534946 Free PMC article. Review.
Cited by
-
Effect of swab pooling on the Accula point-of-care RT-PCR for SARS-CoV-2 detection.Front Microbiol. 2023 Aug 7;14:1219214. doi: 10.3389/fmicb.2023.1219214. eCollection 2023. Front Microbiol. 2023. PMID: 37608952 Free PMC article.
-
Machine Learning Highlights Downtrending of COVID-19 Patients with a Distinct Laboratory Profile.Health Data Sci. 2021 Jun 16;2021:7574903. doi: 10.34133/2021/7574903. eCollection 2021. Health Data Sci. 2021. PMID: 36405356 Free PMC article.
-
Evaluation of conventional and point-of-care real-time RT-PCR tests for the detection of SARS-CoV-2 through a pooled testing strategy.J Clin Lab Anal. 2022 Jun;36(6):e24491. doi: 10.1002/jcla.24491. Epub 2022 May 9. J Clin Lab Anal. 2022. PMID: 35535393 Free PMC article.
-
Taqman PACMAN: a simple molecular approach for positive rapid antigen test confirmation during periods of low prevalence.Microbiol Spectr. 2024 May 2;12(5):e0407323. doi: 10.1128/spectrum.04073-23. Epub 2024 Apr 3. Microbiol Spectr. 2024. PMID: 38567975 Free PMC article.
-
A Pooling Strategy for Detecting Carbapenem Resistance Genes by the Xpert Carba-R Test in Rectal Swab Specimens.J Clin Microbiol. 2022 Dec 21;60(12):e0118122. doi: 10.1128/jcm.01181-22. Epub 2022 Nov 14. J Clin Microbiol. 2022. PMID: 36374075 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

