Macrophage-Derived Vascular Endothelial Growth Factor-A Is Integral to Neuromuscular Junction Reinnervation after Nerve Injury
- PMID: 33158964
- PMCID: PMC7726545
- DOI: 10.1523/JNEUROSCI.1736-20.2020
Macrophage-Derived Vascular Endothelial Growth Factor-A Is Integral to Neuromuscular Junction Reinnervation after Nerve Injury
Abstract
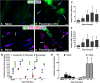
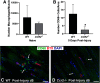
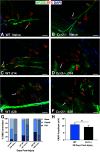
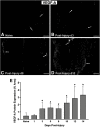
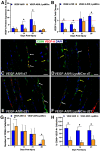
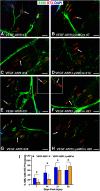
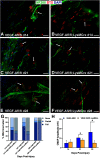
Functional recovery in the end target muscle is a determinant of outcome after peripheral nerve injury. The neuromuscular junction (NMJ) provides the interface between nerve and muscle and includes non-myelinating terminal Schwann cells (tSCs). After nerve injury, tSCs extend cytoplasmic processes between NMJs to guide axon growth and NMJ reinnervation. The mechanisms related to NMJ reinnervation are not known. We used multiple mouse models to investigate the mechanisms of NMJ reinnervation in both sexes, specifically whether macrophage-derived vascular endothelial growth factor-A (Vegf-A) is crucial to establishing NMJ reinnervation at the end target muscle. Both macrophage number and Vegf-A expression increased in end target muscles after nerve injury and repair. In mice with impaired recruitment of macrophages and monocytes (Ccr2-/- mice), the absence of CD68+ cells (macrophages) in the muscle resulted in diminished muscle function. Using a Vegf-receptor 2 (VegfR2) inhibitor (cabozantinib; CBZ) via oral gavage in wild-type (WT) mice resulted in reduced tSC cytoplasmic process extension and decreased NMJ reinnervation compared with saline controls. Mice with Vegf-A conditionally knocked out in macrophages (Vegf-Afl/fl; LysMCre mice) demonstrated a more prolonged detrimental effect on NMJ reinnervation and worse functional muscle recovery. Together, these results show that contributions of the immune system are integral for NMJ reinnervation and functional muscle recovery after nerve injury.SIGNIFICANCE STATEMENT This work demonstrates beneficial contributions of a macrophage-mediated response for neuromuscular junction (NMJ) reinnervation following nerve injury and repair. Macrophage recruitment occurred at the NMJ, distant from the nerve injury site, to support functional recovery at the muscle. We have shown hindered terminal Schwann cell (tSC) injury response and NMJ recovery with inhibition of: (1) macrophage recruitment after injury; (2) vascular endothelial growth factor receptor 2 (VegfR2) signaling; and (3) Vegf secretion from macrophages. We conclude that macrophage-derived Vegf is a key component of NMJ recovery after injury. Determining the mechanisms active at the end target muscle after motor nerve injury reveals new therapeutic targets that may translate to improve motor recovery following nerve injury.
Keywords: muscle recovery; nerve injury; neuromuscular junction; reinnervation; terminal Schwann cell; vascular endothelial growth factor.
Copyright © 2020 the authors.
Figures

Similar articles
-
FK506 Enhancement of Neuromuscular Junction Recovery After Nerve Injury Is Macrophage-Dependent.Muscle Nerve. 2025 Mar;71(3):463-473. doi: 10.1002/mus.28336. Epub 2025 Jan 8. Muscle Nerve. 2025. PMID: 39780562
-
Gpr126/Adgrg6 contributes to the terminal Schwann cell response at the neuromuscular junction following peripheral nerve injury.Glia. 2020 Jun;68(6):1182-1200. doi: 10.1002/glia.23769. Epub 2019 Dec 24. Glia. 2020. PMID: 31873966 Free PMC article.
-
Terminal Schwann Cells Are Essential for Neuromuscular Junction Function and Recovery after Nerve Injury.Plast Reconstr Surg. 2023 Apr 1;151(4):792-803. doi: 10.1097/PRS.0000000000009999. Epub 2022 Dec 6. Plast Reconstr Surg. 2023. PMID: 36729941
-
Macrophage roles in peripheral nervous system injury and pathology: Allies in neuromuscular junction recovery.Mol Cell Neurosci. 2021 Mar;111:103590. doi: 10.1016/j.mcn.2021.103590. Epub 2021 Jan 8. Mol Cell Neurosci. 2021. PMID: 33422671 Free PMC article. Review.
-
Applications of Proteomics to Nerve Regeneration Research.In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. PMID: 21882439 Free Books & Documents. Review.
Cited by
-
Non-Interferon-Dependent Role of STING Signaling in Pulmonary Hypertension.Arterioscler Thromb Vasc Biol. 2024 Jan;44(1):124-142. doi: 10.1161/ATVBAHA.123.320121. Epub 2023 Nov 9. Arterioscler Thromb Vasc Biol. 2024. PMID: 37942608 Free PMC article.
-
Inflammation balance in skeletal muscle damage and repair.Front Immunol. 2023 Jan 26;14:1133355. doi: 10.3389/fimmu.2023.1133355. eCollection 2023. Front Immunol. 2023. PMID: 36776867 Free PMC article. Review.
-
IL-4 expressing cells are recruited to nerve after injury and promote regeneration.Exp Neurol. 2022 Jan;347:113909. doi: 10.1016/j.expneurol.2021.113909. Epub 2021 Oct 28. Exp Neurol. 2022. PMID: 34717939 Free PMC article.
-
25-Hydroxycholesterol as a Signaling Molecule of the Nervous System.Biochemistry (Mosc). 2022 Jun;87(6):524-537. doi: 10.1134/S0006297922060049. Biochemistry (Mosc). 2022. PMID: 35790411 Free PMC article. Review.
-
Ultrasound Stimulation Inhibits Morphological Degeneration of Motor Endplates in the Denervated Skeletal Muscle of Rats.Neurosci Insights. 2022 Nov 19;17:26331055221138508. doi: 10.1177/26331055221138508. eCollection 2022. Neurosci Insights. 2022. PMID: 36420426 Free PMC article.
References
-
- Bogdanik LP, Osborne MA, Davis C, Martin WP, Austin A, Rigo F, Bennett CF, Lutz CM (2015) Systemic, postsymptomatic antisense oligonucleotide rescues motor unit maturation delay in a new mouse model for type II/III spinal muscular atrophy. Proc Natl Acad Sci USA 112:E5863–E5872. 10.1073/pnas.1509758112 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

