Safety and efficacy of artemisinin-piperaquine for treatment of COVID-19: an open-label, non-randomised and controlled trial
- PMID: 33152450
- PMCID: PMC7605811
- DOI: 10.1016/j.ijantimicag.2020.106216
Safety and efficacy of artemisinin-piperaquine for treatment of COVID-19: an open-label, non-randomised and controlled trial
Abstract
Background: There are no effective therapies for patients with coronavirus disease-2019 (COVID-19).
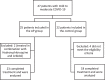
Methods: Forty-one patients with confirmed COVID-19 were enrolled in the study and divided into two groups: artemisinin-piperaquine (AP) (n = 23) and control (n = 18). The primary outcome were the time taken to reach undetectable levels of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) and the percentage of participants with undetectable SARS-CoV-2 on days 7, 10, 14, and 28. The computed tomography (CT) imaging changes within 10 days, corrected QT interval changes, adverse events, and abnormal laboratory parameters were the secondary outcomes.
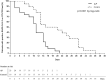
Results: The mean time to reach undetectable viral RNA (mean ± standard deviation) was 10.6 ± 1.1 days (95% confidence interval [CI] 8.4-12.8) for the AP group and 19.3 ± 2.1 days (95% CI 15.1-23.5) for the control group. The percentages of patients with undetectable viral RNA on days 7, 10, 14, 21, and 28 were 26.1%, 43.5%, 78.3%, 100%, and 100%, respectively, in the AP group and 5.6%, 16.7%, 44.4%, 55.6%, and 72.2%, respectively, in the control group. The CT imaging within 10 days post-treatment showed no significant between-group differences (P > 0.05). Both groups had mild adverse events.
Conclusions: In patients with mild-to-moderate COVID-19, the time to reach undetectable SARS-CoV-2 was significantly shorter in the AP group than that in the control group. However, physicians should consider QT interval changes before using AP.
Keywords: Antimalarial; Artemisinin; COVID-19; Piperaquine; SARS-CoV-2.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


Similar articles
-
Triple artemisinin-based combination therapies versus artemisinin-based combination therapies for uncomplicated Plasmodium falciparum malaria: a multicentre, open-label, randomised clinical trial.Lancet. 2020 Apr 25;395(10233):1345-1360. doi: 10.1016/S0140-6736(20)30552-3. Epub 2020 Mar 11. Lancet. 2020. PMID: 32171078 Free PMC article. Clinical Trial.
-
Effect of artemisinin-piperaquine treatment on the electrocardiogram of malaria patients.Rev Soc Bras Med Trop. 2019 May 16;52:e20180453. doi: 10.1590/0037-8682-0453-2018. Rev Soc Bras Med Trop. 2019. PMID: 31141053
-
Early antiviral treatment in outpatients with COVID-19 (FLARE): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Mar 8;22(1):193. doi: 10.1186/s13063-021-05139-2. Trials. 2021. PMID: 33685502 Free PMC article.
-
Dihydroartemisinin/Piperaquine: a review of its use in the treatment of uncomplicated Plasmodium falciparum malaria.Drugs. 2012 May 7;72(7):937-61. doi: 10.2165/11203910-000000000-00000. Drugs. 2012. PMID: 22515619 Review.
-
Dihydroartemisinin-piperaquine for treating uncomplicated Plasmodium falciparum malaria.Cochrane Database Syst Rev. 2014 Jan 20;2014(1):CD010927. doi: 10.1002/14651858.CD010927. Cochrane Database Syst Rev. 2014. PMID: 24443033 Free PMC article. Review.
Cited by
-
Applicability of Redirecting Artemisinins for New Targets.Glob Chall. 2023 Oct 27;7(12):2300030. doi: 10.1002/gch2.202300030. eCollection 2023 Dec. Glob Chall. 2023. PMID: 38094863 Free PMC article. Review.
-
SARS-CoV-2 omicron variants are susceptible in vitro to Artemisia annua hot water extracts.J Ethnopharmacol. 2023 May 23;308:116291. doi: 10.1016/j.jep.2023.116291. Epub 2023 Feb 18. J Ethnopharmacol. 2023. PMID: 36804200 Free PMC article.
-
Current utilization of interferon alpha for the treatment of coronavirus disease 2019: A comprehensive review.Cytokine Growth Factor Rev. 2022 Feb;63:34-43. doi: 10.1016/j.cytogfr.2022.01.001. Epub 2022 Jan 13. Cytokine Growth Factor Rev. 2022. PMID: 35115233 Free PMC article. Review.
-
Chemical Composition, Insecticidal, Persistence and Detoxification Enzyme Inhibition Activities of Essential Oil of Artemisia maritima against the Pulse Beetle.Molecules. 2022 Feb 25;27(5):1547. doi: 10.3390/molecules27051547. Molecules. 2022. PMID: 35268647 Free PMC article.
-
Therapeutic capability of five active compounds in typical African medicinal plants against main proteases of SARS-CoV-2 by computational approach.Inform Med Unlocked. 2022;31:100964. doi: 10.1016/j.imu.2022.100964. Epub 2022 May 23. Inform Med Unlocked. 2022. PMID: 35647264 Free PMC article.
References
-
- WHO. Data from who.int/emergencies/diseases/novel-coronavirus-2019. n.d.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

