Dual Pathways of Human Immunodeficiency Virus Type 1 Envelope Glycoprotein Trafficking Modulate the Selective Exclusion of Uncleaved Oligomers from Virions
- PMID: 33148792
- PMCID: PMC7925103
- DOI: 10.1128/JVI.01369-20
Dual Pathways of Human Immunodeficiency Virus Type 1 Envelope Glycoprotein Trafficking Modulate the Selective Exclusion of Uncleaved Oligomers from Virions
Abstract
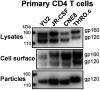
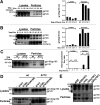
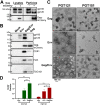
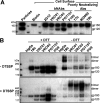
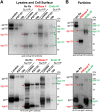
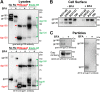
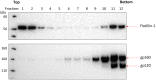
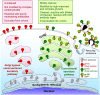
The human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein (Env) trimer is transported through the secretory pathway to the infected cell surface and onto virion particles. In the Golgi, the gp160 Env precursor is modified by complex sugars and proteolytically cleaved to produce the mature functional Env trimer, which resists antibody neutralization. We observed mostly uncleaved gp160 and smaller amounts of cleaved gp120 and gp41 Envs on the surface of HIV-1-infected or Env-expressing cells; however, cleaved Envs were relatively enriched in virions and virus-like particles (VLPs). This relative enrichment of cleaved Env in VLPs was observed for wild-type Envs, for Envs lacking the cytoplasmic tail, and for CD4-independent, conformationally flexible Envs. On the cell surface, we identified three distinct populations of Envs: (i) the cleaved Env was transported through the Golgi, was modified by complex glycans, formed trimers that cross-linked efficiently, and was recognized by broadly neutralizing antibodies; (ii) a small fraction of Env modified by complex carbohydrates escaped cleavage in the Golgi; and (iii) the larger population of uncleaved Env lacked complex carbohydrates, cross-linked into diverse oligomeric forms, and was recognized by poorly neutralizing antibodies. This last group of more "open" Env oligomers reached the cell surface in the presence of brefeldin A, apparently bypassing the Golgi apparatus. Relative to Envs transported through the Golgi, these uncleaved Envs were counterselected for virion incorporation. By employing two pathways for Env transport to the surface of infected cells, HIV-1 can misdirect host antibody responses toward conformationally flexible, uncleaved Env without compromising virus infectivity.IMPORTANCE The envelope glycoprotein (Env) trimers on the surface of human immunodeficiency virus type 1 (HIV-1) mediate the entry of the virus into host cells and serve as targets for neutralizing antibodies. The cleaved, functional Env is incorporated into virus particles from the surface of the infected cell. We found that an uncleaved form of Env is transported to the cell surface by an unconventional route, but this nonfunctional Env is mostly excluded from the virus. Thus, only one of the pathways by which Env is transported to the surface of infected cells results in efficient incorporation into virus particles, potentially allowing the uncleaved Env to act as a decoy to the host immune system without compromising virus infectivity.
Keywords: Env; Golgi bypass; antibody; cell surface; cleavage; trafficking; virion incorporation.
Copyright © 2021 American Society for Microbiology.
Figures

Similar articles
-
Inhibition of human immunodeficiency virus (HIV-1) infectivity by expression of poorly or broadly neutralizing antibodies against Env in virus-producing cells.J Virol. 2024 Feb 20;98(2):e0159423. doi: 10.1128/jvi.01594-23. Epub 2024 Jan 30. J Virol. 2024. PMID: 38289101 Free PMC article.
-
Comparison of Uncleaved and Mature Human Immunodeficiency Virus Membrane Envelope Glycoprotein Trimers.J Virol. 2018 May 29;92(12):e00277-18. doi: 10.1128/JVI.00277-18. Print 2018 Jun 15. J Virol. 2018. PMID: 29618643 Free PMC article.
-
Inducible cell lines producing replication-defective human immunodeficiency virus particles containing envelope glycoproteins stabilized in a pretriggered conformation.J Virol. 2024 Dec 17;98(12):e0172024. doi: 10.1128/jvi.01720-24. Epub 2024 Nov 7. J Virol. 2024. PMID: 39508605
-
HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation.J Mol Biol. 2011 Jul 22;410(4):582-608. doi: 10.1016/j.jmb.2011.04.042. J Mol Biol. 2011. PMID: 21762802 Free PMC article. Review.
-
Immunogenicity of HIV-1 envelope glycoprotein oligomers.Curr Opin HIV AIDS. 2009 Sep;4(5):380-7. doi: 10.1097/COH.0b013e32832edc19. Curr Opin HIV AIDS. 2009. PMID: 20048701 Review.
Cited by
-
Cell-cell communication: new insights and clinical implications.Signal Transduct Target Ther. 2024 Aug 7;9(1):196. doi: 10.1038/s41392-024-01888-z. Signal Transduct Target Ther. 2024. PMID: 39107318 Free PMC article. Review.
-
Single-Molecule FRET Imaging of Virus Spike-Host Interactions.Viruses. 2021 Feb 21;13(2):332. doi: 10.3390/v13020332. Viruses. 2021. PMID: 33669922 Free PMC article. Review.
-
ADCC-mediating non-neutralizing antibodies can exert immune pressure in early HIV-1 infection.PLoS Pathog. 2021 Nov 17;17(11):e1010046. doi: 10.1371/journal.ppat.1010046. eCollection 2021 Nov. PLoS Pathog. 2021. PMID: 34788337 Free PMC article.
-
Spike glycoprotein and host cell determinants of SARS-CoV-2 entry and cytopathic effects.J Virol. 2021 Mar 1;95(5):e02304-20. doi: 10.1128/JVI.02304-20. Epub 2020 Dec 11. J Virol. 2021. PMID: 33310888 Free PMC article.
-
HIV-1 Envelope Glycoproteins Proteolytic Cleavage Protects Infected Cells from ADCC Mediated by Plasma from Infected Individuals.Viruses. 2021 Nov 6;13(11):2236. doi: 10.3390/v13112236. Viruses. 2021. PMID: 34835042 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

