Insulin granule biogenesis and exocytosis
- PMID: 33146746
- PMCID: PMC7966131
- DOI: 10.1007/s00018-020-03688-4
Insulin granule biogenesis and exocytosis
Abstract
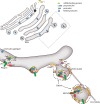
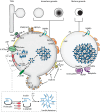
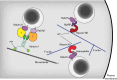
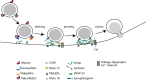
Insulin is produced by pancreatic β-cells, and once released to the blood, the hormone stimulates glucose uptake and suppresses glucose production. Defects in both the availability and action of insulin lead to elevated plasma glucose levels and are major hallmarks of type-2 diabetes. Insulin is stored in secretory granules that form at the trans-Golgi network. The granules undergo extensive modifications en route to their release sites at the plasma membrane, including changes in both protein and lipid composition of the granule membrane and lumen. In parallel, the insulin molecules also undergo extensive modifications that render the hormone biologically active. In this review, we summarize current understanding of insulin secretory granule biogenesis, maturation, transport, docking, priming and eventual fusion with the plasma membrane. We discuss how different pools of granules form and how these pools contribute to insulin secretion under different conditions. We also highlight the role of the β-cell in the development of type-2 diabetes and discuss how dysregulation of one or several steps in the insulin granule life cycle may contribute to disease development or progression.
Keywords: Diabetes; Insulin; Lipids; β-Cell.
Figures
Similar articles
-
Deploying insulin granule-granule fusion to rescue deficient insulin secretion in diabetes.Diabetologia. 2012 Apr;55(4):877-80. doi: 10.1007/s00125-012-2483-7. Epub 2012 Feb 4. Diabetologia. 2012. PMID: 22307686
-
Mathematical modeling of insulin secretion and the role of glucose-dependent mobilization, docking, priming and fusion of insulin granules.J Theor Biol. 2013 Feb 7;318:210-25. doi: 10.1016/j.jtbi.2012.11.002. Epub 2012 Nov 12. J Theor Biol. 2013. PMID: 23154190 Free PMC article.
-
Kv2.1 Clustering Contributes to Insulin Exocytosis and Rescues Human β-Cell Dysfunction.Diabetes. 2017 Jul;66(7):1890-1900. doi: 10.2337/db16-1170. Epub 2017 Jun 12. Diabetes. 2017. PMID: 28607108 Free PMC article.
-
Recent new insights into the role of SNARE and associated proteins in insulin granule exocytosis.Diabetes Obes Metab. 2017 Sep;19 Suppl 1:115-123. doi: 10.1111/dom.13001. Diabetes Obes Metab. 2017. PMID: 28880475 Review.
-
Insulin granule biogenesis, trafficking and exocytosis.Vitam Horm. 2009;80:473-506. doi: 10.1016/S0083-6729(08)00616-X. Vitam Horm. 2009. PMID: 19251047 Free PMC article. Review.
Cited by
-
Lipid Droplets' Role in the Regulation of β-Cell Function and β-Cell Demise in Type 2 Diabetes.Endocrinology. 2022 Mar 1;163(3):bqac007. doi: 10.1210/endocr/bqac007. Endocrinology. 2022. PMID: 35086144 Free PMC article. Review.
-
Dysfunctions, molecular mechanisms, and therapeutic strategies of pancreatic β-cells in diabetes.Apoptosis. 2023 Aug;28(7-8):958-976. doi: 10.1007/s10495-023-01854-0. Epub 2023 Jun 5. Apoptosis. 2023. PMID: 37273039 Review.
-
Genome-wide association analysis identifies ancestry-specific genetic variation associated with acute response to metformin and glipizide in SUGAR-MGH.Diabetologia. 2023 Jul;66(7):1260-1272. doi: 10.1007/s00125-023-05922-7. Epub 2023 May 26. Diabetologia. 2023. PMID: 37233759 Free PMC article.
-
Chronic binge alcohol and ovariectomy-mediated impaired insulin responsiveness in SIV-infected female rhesus macaques.Am J Physiol Regul Integr Comp Physiol. 2021 Nov 1;321(5):R699-R711. doi: 10.1152/ajpregu.00159.2021. Epub 2021 Sep 15. Am J Physiol Regul Integr Comp Physiol. 2021. PMID: 34524906 Free PMC article.
-
The insulin secretory granule is a hotspot for autoantigen formation in type 1 diabetes.Diabetologia. 2024 Aug;67(8):1507-1516. doi: 10.1007/s00125-024-06164-x. Epub 2024 May 29. Diabetologia. 2024. PMID: 38811417 Review.
References
-
- Andrali SS, Sampley ML, Vanderford NL, Ozcan S. Glucose regulation of insulin gene expression in pancreatic beta-cells. Biochem J. 2008;415(1):1–10. - PubMed
-
- Tillmar L, Carlsson C, Welsh N. Control of insulin mRNA stability in rat pancreatic islets. Regulatory role of a 3'-untranslated region pyrimidine-rich sequence. J Biol Chem. 2002;277(2):1099–1106. - PubMed
-
- Dodson G, Steiner D. The role of assembly in insulin's biosynthesis. Curr Opin Struct Biol. 1998;8(2):189–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

