Congenital muscular dystrophy-associated inflammatory chemokines provide axes for effective recruitment of therapeutic adult stem cell into muscles
- PMID: 33138863
- PMCID: PMC7607684
- DOI: 10.1186/s13287-020-01979-y
Congenital muscular dystrophy-associated inflammatory chemokines provide axes for effective recruitment of therapeutic adult stem cell into muscles
Abstract
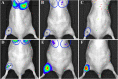
Background: Congenital muscular dystrophies (CMD) are a clinically and genetically heterogeneous group of neuromuscular disorders characterized by muscle weakness. The two most prevalent forms of CMD, collagen VI-related myopathies (COL6RM) and laminin α2 deficient CMD type 1A (MDC1A), are both caused by deficiency or dysfunction of extracellular matrix proteins. Previously, we showed that an intramuscular transplantation of human adipose-derived stem cells (ADSC) into the muscle of the Col6a1-/- mice results in efficient stem cell engraftment, migration, long-term survival, and continuous production of the collagen VI protein, suggesting the feasibility of the systemic cellular therapy for COL6RM. In order for this therapeutic approach to work however, stem cells must be efficiently targeted to the entire body musculature. Thus, the main goal of this study is to test whether muscle homing of systemically transplanted ADSC can be enhanced by employing muscle-specific chemotactic signals originating from CMD-affected muscle tissue.
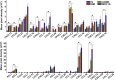
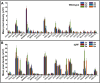
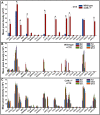
Methods: Proteomic screens of chemotactic molecules were conducted in the skeletal muscles of COL6RM- and MDC1A-affected patients and CMD mouse models to define the inflammatory and immune activities, thus, providing potential markers of disease activity or treatment effect. Also using a pre-clinical animal model, recapitulating mild Ullrich congenital muscular dystrophy (UCMD), the therapeutic relevance of identified chemotactic pathways was investigated in vivo, providing a basis for future clinical investigations.
Results: Comprehensive proteomic screens evaluating relevant human and mouse skeletal muscle biopsies offered chemotactic axes to enhance directional migration of systemically transplanted cells into CMD-affected muscles, including CCL5-CCR1/3/5, CCL2-CCR2, CXCL1/2-CXCR1,2, and CXCL7-CXCR2. Also, the specific populations of ADSC selected with an affinity for the chemokines being released by damaged muscle showed efficient migration to injured site and presented their therapeutic effect.
Conclusions: Collectively, identified molecules provided insight into the mechanisms governing directional migration and intramuscular trafficking of systemically infused stem cells, thus, permitting broad and effective application of the therapeutic adult stem cells for CMD treatment.
Keywords: Adipose-derived stem cell; COL6A1; Cell-based therapy; Chemokines; Chemotaxis; Congenital muscular dystrophy; Type VI collagen.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Human adipose-derived stem cell transplantation as a potential therapy for collagen VI-related congenital muscular dystrophy.Stem Cell Res Ther. 2014 Feb 12;5(1):21. doi: 10.1186/scrt411. Stem Cell Res Ther. 2014. PMID: 24522088 Free PMC article.
-
Chemotaxis-driven disease-site targeting of therapeutic adult stem cells in dystrophic epidermolysis bullosa.Stem Cell Res Ther. 2016 Aug 27;7(1):124. doi: 10.1186/s13287-016-0388-y. Stem Cell Res Ther. 2016. PMID: 27568180 Free PMC article.
-
Distinct muscle regenerative capacity of human induced pluripotent stem cell-derived mesenchymal stromal cells in Ullrich congenital muscular dystrophy model mice.Stem Cell Res Ther. 2024 Oct 7;15(1):340. doi: 10.1186/s13287-024-03951-6. Stem Cell Res Ther. 2024. PMID: 39370505 Free PMC article.
-
Congenital muscular dystrophy. Part I: a review of phenotypical and diagnostic aspects.Arq Neuropsiquiatr. 2009 Mar;67(1):144-68. doi: 10.1590/s0004-282x2009000100038. Arq Neuropsiquiatr. 2009. PMID: 19330236 Review.
-
Congenital muscular dystrophy. Part II: a review of pathogenesis and therapeutic perspectives.Arq Neuropsiquiatr. 2009 Jun;67(2A):343-62. doi: 10.1590/s0004-282x2009000200035. Arq Neuropsiquiatr. 2009. PMID: 19547838 Review.
Cited by
-
Muscle eosinophilia is a hallmark of chronic disease in facioscapulohumeral muscular dystrophy.Hum Mol Genet. 2024 May 4;33(10):872-883. doi: 10.1093/hmg/ddae019. Hum Mol Genet. 2024. PMID: 38340007 Free PMC article.
-
Adipose-derived stem cells therapy effectively attenuates PM2.5-induced lung injury.Stem Cell Res Ther. 2021 Jun 19;12(1):355. doi: 10.1186/s13287-021-02441-3. Stem Cell Res Ther. 2021. PMID: 34147136 Free PMC article.
-
Nano-Immunomodulation: A New Strategy for Skeletal Muscle Diseases and Aging?Int J Mol Sci. 2023 Jan 7;24(2):1175. doi: 10.3390/ijms24021175. Int J Mol Sci. 2023. PMID: 36674691 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

