Electro-Hydrodynamic Drop-on-Demand Printing of Aqueous Suspensions of Drug Nanoparticles
- PMID: 33138033
- PMCID: PMC7693662
- DOI: 10.3390/pharmaceutics12111034
Electro-Hydrodynamic Drop-on-Demand Printing of Aqueous Suspensions of Drug Nanoparticles
Abstract
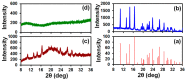
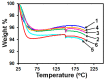
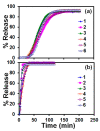
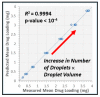
We demonstrate the ability to fabricate dosage forms of a poorly water-soluble drug by using wet stirred media milling of a drug powder to produce an aqueous suspension of nanoparticles and then print it onto a porous biocompatible film. Contrary to conventional printing technologies, a deposited material is pulled out from the nozzle. This feature enables printing highly viscous materials with a precise control over the printed volume. Drug (griseofulvin) nanosuspensions prepared by wet media milling were printed onto porous hydroxypropyl methylcellulose films prepared by freeze-drying. The drug particles retained crystallinity and polymorphic form in the course of milling and printing. The versatility of this technique was demonstrated by printing the same amount of nanoparticles onto a film with droplets of different sizes. The mean drug content (0.19-3.80 mg) in the printed films was predicted by the number of droplets (5-100) and droplet volume (0.2-1.0 µL) (R2 = 0.9994, p-value < 10-4). Our results also suggest that for any targeted drug content, the number-volume of droplets could be modulated to achieve acceptable drug content uniformity. Analysis of the model-independent difference and similarity factors showed consistency of drug release profiles from films with a printed suspension. Zero-order kinetics described the griseofulvin release rate from 1.8% up to 82%. Overall, this study has successfully demonstrated that the electro-hydrodynamic drop-on-demand printing of an aqueous drug nanosuspension enables accurate and controllable drug dosing in porous polymer films, which exhibited acceptable content uniformity and reproducible drug release.
Keywords: biocompatible films; drop-on-demand printing; drug release profile; nanoparticles; poorly water-soluble drugs; precision dosage form.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Fast drying of biocompatible polymer films loaded with poorly water-soluble drug nano-particles via low temperature forced convection.Int J Pharm. 2013 Oct 15;455(1-2):93-103. doi: 10.1016/j.ijpharm.2013.07.051. Epub 2013 Jul 31. Int J Pharm. 2013. PMID: 23911341
-
Critical material attributes (CMAs) of strip films loaded with poorly water-soluble drug nanoparticles: I. Impact of plasticizer on film properties and dissolution.Eur J Pharm Sci. 2016 Sep 20;92:146-55. doi: 10.1016/j.ejps.2016.07.005. Epub 2016 Jul 8. Eur J Pharm Sci. 2016. PMID: 27402100 Free PMC article.
-
Critical material attributes (CMAs) of strip films loaded with poorly water-soluble drug nanoparticles: III. Impact of drug nanoparticle loading.Int J Pharm. 2017 May 15;523(1):33-41. doi: 10.1016/j.ijpharm.2017.03.023. Epub 2017 Mar 16. Int J Pharm. 2017. PMID: 28315716 Free PMC article.
-
Critical Material Attributes of Strip Films Loaded With Poorly Water-Soluble Drug Nanoparticles: II. Impact of Polymer Molecular Weight.J Pharm Sci. 2017 Feb;106(2):619-628. doi: 10.1016/j.xphs.2016.10.009. Epub 2016 Nov 18. J Pharm Sci. 2017. PMID: 27871727
-
Preparation and characterization of fast dissolving pullulan films containing BCS class II drug nanoparticles for bioavailability enhancement.Drug Dev Ind Pharm. 2016;42(7):1073-85. doi: 10.3109/03639045.2015.1107094. Epub 2015 Nov 15. Drug Dev Ind Pharm. 2016. PMID: 26567632
References
-
- Personalized Medicine Coalition Personalized Medicine at FDA: The Scope & Significance of Progress in 2019. [(accessed on 6 September 2020)]; Available online: http://www.personalizedmedicinecoalition.org/Resources/Personalized_Medi....
Grants and funding
LinkOut - more resources
Full Text Sources

