New directions for emerging therapies in acute myeloid leukemia: the next chapter
- PMID: 33127875
- PMCID: PMC7599225
- DOI: 10.1038/s41408-020-00376-1
New directions for emerging therapies in acute myeloid leukemia: the next chapter
Abstract
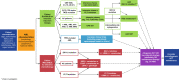
Conventional therapy for acute myeloid leukemia is composed of remission induction with cytarabine- and anthracycline-containing regimens, followed by consolidation therapy, including allogeneic stem cell transplantation, to prolong remission. In recent years, there has been a significant shift toward the use of novel and effective, target-directed therapies, including inhibitors of mutant FMS-like tyrosine kinase 3 (FLT3) and isocitrate dehydrogenase (IDH), the B-cell lymphoma 2 inhibitor venetoclax, and the hedgehog pathway inhibitor glasdegib. In older patients the combination of a hypomethylating agent or low-dose cytarabine, venetoclax achieved composite response rates that approximate those seen with standard induction regimens in similar populations, but with potentially less toxicity and early mortality. Preclinical data suggest synergy between venetoclax and FLT3- and IDH-targeted therapies, and doublets of venetoclax with inhibitors targeting these mutations have shown promising clinical activity in early stage trials. Triplet regimens involving the hypomethylating agent and venetoclax with FLT3 or IDH1/2 inhibitor, the TP53-modulating agent APR-246 and magrolimab, myeloid cell leukemia-1 inhibitors, or immune therapies such as CD123 antibody-drug conjugates and programmed cell death protein 1 inhibitors are currently being evaluated. It is hoped that such triplets, when applied in appropriate patient subsets, will further enhance remission rates, and more importantly remission durations and survival.
Conflict of interest statement
A.H.W.: Honoraria from Amgen, Servier, Novartis, Celgene, AbbVie/Genentech, Roche, Pfizer, Janssen Oncology; consulting or advisory role for Servier, Novartis, Amgen, AbbVie/Genentech; speakers’ bureau for AbbVie/Genentech, Novartis; research funding from Novartis (institution), Celgene (institution); former employee of Walter and Eliza Hall Institute, which receives milestone and royalty payments related to venetoclax, and Dr Wei is eligible for benefits related to these payments. D.A.P.: Consulting or advisory role for Celyad, Pfizer, AbbVie, Gilead, Astellas, Forty Seven, Daiichi Sankyo, Celgene, Janssen, Takeda, Amgen; research funding from AbbVie and Pfizer. A.T.F.: Consulting or advisory role for Agios, Seattle Genetics, Forty Seven, Amphivena, AbbVie, Amgen, Astellas, Celgene (BMS), Clear Creek Bio, Trovagene, Novartis, Daiichi Sankyo, Takeda; clinical trial support from Takeda, Celgene, Exelixis, Seattle Genetics. P.V.: Consulting or advisory role for Celgene, Novartis, Jazz, Agios, Pfizer, AbbVie, Astellas, Daiichi Sankyo, Janssen, Takeda; research funding from Celgene, Novartis; scientific advisory board for Auron; co-founder, Oxstem Oncology. C.D.DiNardo: Consulting or advisory role for AbbVie, Agios, Celgene, Daiichi Sankyo, Jazz, Notable Labs; research funding from AbbVie, Agios, Celgene, Daiichi Sankyo, Calithera, Clear Creek Bio. N.D.: Research funding from Daiichi Sankyo, Bristol-Myers Squibb, Pfizer, Karyopharm, Servier, Genentech, ImmunoGen, Novimmune, Incyte, AbbVie, Astellas, Forty Seven; consulting or advisory role for Daiichi Sankyo, Bristol-Myers Squibb, Pfizer, Novartis, Celgene, AbbVie, Agios, Astellas, Genentech, Immunogen, Jazz, Forty Seven, Trovagene, Gilead.
Figures
Similar articles
-
Venetoclax-based therapies for acute myeloid leukemia.Best Pract Res Clin Haematol. 2019 Jun;32(2):145-153. doi: 10.1016/j.beha.2019.05.008. Epub 2019 May 24. Best Pract Res Clin Haematol. 2019. PMID: 31203996 Free PMC article. Review.
-
New drugs approved for acute myeloid leukaemia in 2018.Br J Clin Pharmacol. 2019 Dec;85(12):2689-2693. doi: 10.1111/bcp.14105. Epub 2019 Dec 13. Br J Clin Pharmacol. 2019. PMID: 31469910 Free PMC article. Review.
-
[New therapeutic agents for acute myeloid leukemia].Rinsho Ketsueki. 2019;60(9):1108-1119. doi: 10.11406/rinketsu.60.1108. Rinsho Ketsueki. 2019. PMID: 31597834 Review. Japanese.
-
Acute myeloid leukemia: Treatment and research outlook for 2021 and the MD Anderson approach.Cancer. 2021 Apr 15;127(8):1186-1207. doi: 10.1002/cncr.33477. Epub 2021 Mar 18. Cancer. 2021. PMID: 33734442 Review.
-
Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study.Lancet Oncol. 2018 Feb;19(2):216-228. doi: 10.1016/S1470-2045(18)30010-X. Epub 2018 Jan 12. Lancet Oncol. 2018. PMID: 29339097 Clinical Trial.
Cited by
-
Pivekimab sunirine (IMGN632), a novel CD123-targeting antibody-drug conjugate, in relapsed or refractory acute myeloid leukaemia: a phase 1/2 study.Lancet Oncol. 2024 Mar;25(3):388-399. doi: 10.1016/S1470-2045(23)00674-5. Lancet Oncol. 2024. PMID: 38423051 Clinical Trial.
-
PD-1H/VISTA mediates immune evasion in acute myeloid leukemia.J Clin Invest. 2024 Feb 1;134(3):e164325. doi: 10.1172/JCI164325. J Clin Invest. 2024. PMID: 38060328 Free PMC article.
-
Survivin' Acute Myeloid Leukaemia-A Personalised Target for inv(16) Patients.Int J Mol Sci. 2021 Sep 28;22(19):10482. doi: 10.3390/ijms221910482. Int J Mol Sci. 2021. PMID: 34638823 Free PMC article.
-
Anti-leukemia effects of omipalisib in acute myeloid leukemia: inhibition of PI3K/AKT/mTOR signaling and suppression of mitochondrial biogenesis.Cancer Gene Ther. 2023 Dec;30(12):1691-1701. doi: 10.1038/s41417-023-00675-2. Epub 2023 Oct 11. Cancer Gene Ther. 2023. PMID: 37821641
-
Lymphocyte Exhaustion in AML Patients and Impacts of HMA/Venetoclax or Intensive Chemotherapy on Their Biology.Cancers (Basel). 2022 Jul 10;14(14):3352. doi: 10.3390/cancers14143352. Cancers (Basel). 2022. PMID: 35884414 Free PMC article.
References
-
- Wiese M, Daver N. Unmet clinical needs and economic burden of disease in the treatment landscape of acute myeloid leukemia. Am. J. Manag. Care. 2018;24:S347–S355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

