Fibroblast growth factor 2 accelerates the epithelial-mesenchymal transition in keratinocytes during wound healing process
- PMID: 33122782
- PMCID: PMC7596476
- DOI: 10.1038/s41598-020-75584-7
Fibroblast growth factor 2 accelerates the epithelial-mesenchymal transition in keratinocytes during wound healing process
Abstract
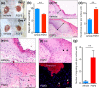
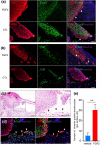
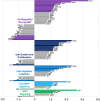
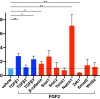
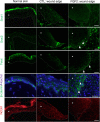
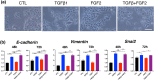
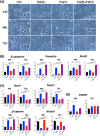
In the wound healing process, the morphology of keratinocytes at the wound edge temporarily changes to a spindle morphology, which is thought to occur due to an epithelial-mesenchymal transition (EMT). Fibroblast growth factor (FGF) 2, also called basic FGF, has the potential to accelerate wound closure by activating vascular endothelial cells and fibroblasts. We examined the effects of FGF2 on keratinocyte morphology and EMT in wounded skin. Histological examination of murine wounds treated with FGF2 revealed that wound edge keratinocytes formed thickened and multilayered epithelia. In addition, we detected wound edge keratinocytes migrating individually toward the wound center. These migrating keratinocytes exhibited not only spindle morphology but also down-regulated E-cadherin and up-regulated vimentin expression, which is characteristic of EMT. In FGF2-treated wounds, a PCR array revealed the upregulation of genes related to EMT, including transforming growth factor (TGF) signaling. Further, FGF2-treated wound edge keratinocytes expressed EMT-associated transcription factors, including Snai2, and showed translocation of β-catenin from the cell membrane to the cytoplasm/nucleus. However, in vitro examination of keratinocytes revealed that FGF2 alone did not activate EMT in keratinocytes, but that FGF2 might promote EMT in combination with TGFβ1. These findings suggest that FGF2 treatment of wounds could promote keratinocyte EMT, accelerating wound closure.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Electric field-induced suppression of PTEN drives epithelial-to-mesenchymal transition via mTORC1 activation.J Dermatol Sci. 2017 Feb;85(2):96-105. doi: 10.1016/j.jdermsci.2016.11.007. Epub 2016 Nov 18. J Dermatol Sci. 2017. PMID: 27919618
-
Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-Slug signaling.Proc Natl Acad Sci U S A. 2016 Jul 26;113(30):E4320-7. doi: 10.1073/pnas.1519197113. Epub 2016 Jul 8. Proc Natl Acad Sci U S A. 2016. PMID: 27466403 Free PMC article.
-
Fibroblast Growth Factor 2 Augments Transforming Growth Factor Beta 1 Induced Epithelial-mesenchymal Transition in Lung Cell Culture Model.Iran J Allergy Asthma Immunol. 2020 Aug 25;19(4):348-361. doi: 10.18502/ijaai.v19i4.4110. Iran J Allergy Asthma Immunol. 2020. PMID: 33463102 Free PMC article.
-
Epithelial-mesenchymal transition, TGF-β, and osteopontin in wound healing and tissue remodeling after injury.J Burn Care Res. 2012 May-Jun;33(3):311-8. doi: 10.1097/BCR.0b013e318240541e. J Burn Care Res. 2012. PMID: 22561306 Free PMC article. Review.
-
Transforming growth factor-beta and wound healing.Perspect Vasc Surg Endovasc Ther. 2006 Mar;18(1):55-62. doi: 10.1177/153100350601800123. Perspect Vasc Surg Endovasc Ther. 2006. PMID: 16628336 Review.
Cited by
-
Structural and biochemical investigation into stable FGF2 mutants with novel mutation sites and hydrophobic replacements for surface-exposed cysteines.PLoS One. 2024 Sep 5;19(9):e0307499. doi: 10.1371/journal.pone.0307499. eCollection 2024. PLoS One. 2024. PMID: 39236042 Free PMC article.
-
Negative-Pressure Wound Therapy: What We Know and What We Need to Know.Adv Exp Med Biol. 2023;1436:131-152. doi: 10.1007/5584_2023_773. Adv Exp Med Biol. 2023. PMID: 36922487
-
Tissue Engineering-Based Strategies for Diabetic Foot Ulcer Management.Adv Wound Care (New Rochelle). 2023 Mar;12(3):145-167. doi: 10.1089/wound.2021.0081. Epub 2021 Dec 22. Adv Wound Care (New Rochelle). 2023. PMID: 34939837 Free PMC article. Review.
-
Growth Factors, Reactive Oxygen Species, and Metformin-Promoters of the Wound Healing Process in Burns?Int J Mol Sci. 2021 Sep 1;22(17):9512. doi: 10.3390/ijms22179512. Int J Mol Sci. 2021. PMID: 34502429 Free PMC article. Review.
-
Double-modified, thio and methylene ATP analogue facilitates wound healing in vitro and in vivo.Sci Rep. 2024 Jun 7;14(1):13148. doi: 10.1038/s41598-024-63759-5. Sci Rep. 2024. PMID: 38849425 Free PMC article.
References
-
- Grinnell F. Wound repair, keratinocyte activation and integrin modulation. J. Cell Sci. 1992;101(Pt 1):1–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

