The Differential and Dynamic Progression of Hepatic Inflammation and Immune Responses During Liver Fibrosis Induced by Schistosoma japonicum or Carbon Tetrachloride in Mice
- PMID: 33117360
- PMCID: PMC7575768
- DOI: 10.3389/fimmu.2020.570524
The Differential and Dynamic Progression of Hepatic Inflammation and Immune Responses During Liver Fibrosis Induced by Schistosoma japonicum or Carbon Tetrachloride in Mice
Abstract
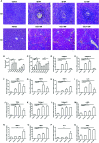
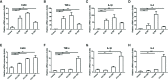
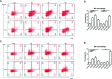
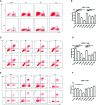
Liver fibrosis can result from various causes and could progress to cirrhosis and cancer; however, there are no effective treatments due to that its molecular mechanism is unclear. liver fibrosis model made by Schistosoma japonicum (S. japonicum) infection or Carbon tetrachloride (CCl4) intraperitoneal injection is a conventional model used in liver fibrosis-related studies for mechanism or pharmaceutical research purposes. But the differences in the pathological progression, immune responses and the underlying mechanism between the two liver fibrosis model have not been carefully compared and characterized, which hinders us from correctly understanding and making better use of the two models. In the present study, the pathological changes to the liver, and the cytokines, inflammatory factors, macrophages, and lymphocytes subsets involved were analyzed in the liver fibrosis model of S. japonicum infection or CCl4 intraperitoneal injection. Additionally, the pathological progression, immune responses and the underlying injury mechanism in these two models were compared and characterized. The results showed that the changing trend of interleukin-13 (IL-13), transforming growth factor beta (TGF-β), inflammatory factors, and M1, M2 macrophages, were consistent with the development trend of fibrosis regardless of whether liver fibrosis was caused by S. japonicum or CCl4. For lymphocyte subsets, the proportions of CD3+ T cells and CD4+ T cells decreased gradually, while proportion of CD8+ T cells peaked at 6 weeks in mice infected with S. japonicum and at 12 weeks in mice injected with CCl4. With prolonged S. japonicum infection time, Th1 (CD4+IFN-γ+) immunity converted to Th2 (CD4+IL-4+)/Th17 (CD4+IL-17+) with weaker regulatory T cell (Treg) (CD4+CD25+FOXP3+) immunity. However, in liver fibrosis caused by CCl4, Th1 cells occupied the dominant position, while proportions of Th2, Th17, and Treg cells decreased gradually. In conclusion, liver fibrosis was a complex pathological process that was regulated by a series of cytokines and immune cells. The pathological progressions and immune responses to S. japonicum or CCl4 induced liver fibrosis were different, possibly because of their different injury mechanisms. The appropriate animal model should be selected according to the needs of different experiments and the pathogenic factors of liver fibrosis in the study.
Keywords: Schistosoma japonicum; carbon tetrachloride; differential progression; dynamic progression; hepatic inflammation; immune response; liver fibrosis.
Copyright © 2020 Song, Yin, Mu, Li, Gao, Zhang, Dong, Mei and Hua.
Figures

Similar articles
-
Characteristics of IL-17 induction by Schistosoma japonicum infection in C57BL/6 mouse liver.Immunology. 2013 Aug;139(4):523-32. doi: 10.1111/imm.12105. Immunology. 2013. PMID: 23551262 Free PMC article.
-
Characteristics of Schistosoma japonicum infection induced IFN-γ and IL-4 co-expressing plasticity Th cells.Immunology. 2016 Sep;149(1):25-34. doi: 10.1111/imm.12623. Immunology. 2016. PMID: 27242265 Free PMC article.
-
T Lymphocyte-Mediated Liver Immunopathology of Schistosomiasis.Front Immunol. 2020 Feb 18;11:61. doi: 10.3389/fimmu.2020.00061. eCollection 2020. Front Immunol. 2020. PMID: 32132991 Free PMC article. Review.
-
[Dynamic alteration of CD154/CD40 and its effects on Th1/Th2 polarization in inducible co-stimulator ligand knockout mice infected with Schistosoma japonicum].Beijing Da Xue Xue Bao Yi Xue Ban. 2015 Dec 18;47(6):898-904. Beijing Da Xue Xue Bao Yi Xue Ban. 2015. PMID: 26679647 Chinese.
-
Pathology and molecular mechanisms of Schistosoma japonicum-associated liver fibrosis.Front Cell Infect Microbiol. 2022 Oct 28;12:1035765. doi: 10.3389/fcimb.2022.1035765. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36389166 Free PMC article. Review.
Cited by
-
Blocking BAFF Alleviates Hepatic Fibrosis in Schistosoma japonicum-Infected Mice.Pathogens. 2023 Jun 1;12(6):793. doi: 10.3390/pathogens12060793. Pathogens. 2023. PMID: 37375483 Free PMC article.
-
Schistosoma japonicum infection-mediated downregulation of lncRNA Malat1 contributes to schistosomiasis hepatic fibrosis by the Malat1/miR-96/Smad7 pathway.Parasit Vectors. 2024 Oct 3;17(1):413. doi: 10.1186/s13071-024-06499-9. Parasit Vectors. 2024. PMID: 39363237 Free PMC article.
-
Kinsenoside attenuates liver fibro-inflammation by suppressing dendritic cells via the PI3K-AKT-FoxO1 pathway.Pharmacol Res. 2022 Mar;177:106092. doi: 10.1016/j.phrs.2022.106092. Epub 2022 Jan 21. Pharmacol Res. 2022. PMID: 35066108 Free PMC article.
-
Sja-Let-7 Attenuates Carbon Tetrachloride-Induced Liver Fibrosis in a Mouse Model via Col1α2.Biology (Basel). 2023 Nov 24;12(12):1465. doi: 10.3390/biology12121465. Biology (Basel). 2023. PMID: 38132291 Free PMC article.
-
Deficiency of PKCλ/ι alleviates the liver pathologic impairment of Schistosoma japonicum infection by thwarting Th2 response.Parasit Vectors. 2022 May 3;15(1):154. doi: 10.1186/s13071-022-05283-x. Parasit Vectors. 2022. PMID: 35505421 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

