Vaccinia Virus Activation and Antagonism of Cytosolic DNA Sensing
- PMID: 33117352
- PMCID: PMC7559579
- DOI: 10.3389/fimmu.2020.568412
Vaccinia Virus Activation and Antagonism of Cytosolic DNA Sensing
Abstract
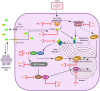
Cells express multiple molecules aimed at detecting incoming virus and infection. Recognition of virus infection leads to the production of cytokines, chemokines and restriction factors that limit virus replication and activate an adaptive immune response offering long-term protection. Recognition of cytosolic DNA has become a central immune sensing mechanism involved in infection, autoinflammation, and cancer immunotherapy. Vaccinia virus (VACV) is the prototypic member of the family Poxviridae and the vaccine used to eradicate smallpox. VACV harbors enormous potential as a vaccine vector and several attenuated strains are currently being developed against infectious diseases. In addition, VACV has emerged as a popular oncolytic agent due to its cytotoxic capacity even in hypoxic environments. As a poxvirus, VACV is an unusual virus that replicates its large DNA genome exclusively in the cytoplasm of infected cells. Despite producing large amounts of cytosolic DNA, VACV efficiently suppresses the subsequent innate immune response by deploying an arsenal of proteins with capacity to disable host antiviral signaling, some of which specifically target cytosolic DNA sensing pathways. Some of these strategies are conserved amongst orthopoxviruses, whereas others are seemingly unique to VACV. In this review we provide an overview of the VACV replicative cycle and discuss the recent advances on our understanding of how VACV induces and antagonizes innate immune activation via cytosolic DNA sensing pathways. The implications of these findings in the rational design of vaccines and oncolytics based on VACV are also discussed.
Keywords: CGAS; STING; antiviral signaling; interferons; vaccinia virus (VACV).
Copyright © 2020 El-Jesr, Teir and Maluquer de Motes.
Figures
Similar articles
-
Virulent Poxviruses Inhibit DNA Sensing by Preventing STING Activation.J Virol. 2018 Apr 27;92(10):e02145-17. doi: 10.1128/JVI.02145-17. Print 2018 May 15. J Virol. 2018. PMID: 29491158 Free PMC article.
-
Human FAM111A inhibits vaccinia virus replication by degrading viral protein I3 and is antagonized by poxvirus host range factor SPI-1.Proc Natl Acad Sci U S A. 2023 Aug 29;120(35):e2304242120. doi: 10.1073/pnas.2304242120. Epub 2023 Aug 22. Proc Natl Acad Sci U S A. 2023. PMID: 37607234 Free PMC article.
-
Type I interferon-dependent CCL4 is induced by a cGAS/STING pathway that bypasses viral inhibition and protects infected tissue, independent of viral burden.PLoS Pathog. 2019 Oct 11;15(10):e1007778. doi: 10.1371/journal.ppat.1007778. eCollection 2019 Oct. PLoS Pathog. 2019. PMID: 31603920 Free PMC article.
-
A vaccinia virus renaissance: new vaccine and immunotherapeutic uses after smallpox eradication.Hum Vaccin Immunother. 2012 Jul;8(7):961-70. doi: 10.4161/hv.21080. Epub 2012 Jul 1. Hum Vaccin Immunother. 2012. PMID: 22777090 Free PMC article. Review.
-
Vaccinia Virus Protein C6: A Multifunctional Interferon Antagonist.Adv Exp Med Biol. 2018;1052:1-7. doi: 10.1007/978-981-10-7572-8_1. Adv Exp Med Biol. 2018. PMID: 29785476 Review.
Cited by
-
Small Hero with Great Powers: Vaccinia Virus E3 Protein and Evasion of the Type I IFN Response.Biomedicines. 2022 Jan 22;10(2):235. doi: 10.3390/biomedicines10020235. Biomedicines. 2022. PMID: 35203445 Free PMC article. Review.
-
Vaccinia E5 is a major inhibitor of the DNA sensor cGAS.Nat Commun. 2023 May 22;14(1):2898. doi: 10.1038/s41467-023-38514-5. Nat Commun. 2023. PMID: 37217469 Free PMC article.
-
ZBP1-Mediated Necroptosis: Mechanisms and Therapeutic Implications.Molecules. 2022 Dec 21;28(1):52. doi: 10.3390/molecules28010052. Molecules. 2022. PMID: 36615244 Free PMC article. Review.
-
NF-κB as an Important Factor in Optimizing Poxvirus-Based Vaccines against Viral Infections.Pathogens. 2020 Nov 29;9(12):1001. doi: 10.3390/pathogens9121001. Pathogens. 2020. PMID: 33260450 Free PMC article. Review.
-
Innate Immune Sensing of Viruses and Its Consequences for the Central Nervous System.Viruses. 2021 Jan 23;13(2):170. doi: 10.3390/v13020170. Viruses. 2021. PMID: 33498715 Free PMC article. Review.
References
-
- Moss B. Poxviridae: the viruses and their replication. In: Knipe DM, editor. Fields Virology. Philadelphia, PA: Lippincott Williams and Wilkins; (2007).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

