SARS-CoV-2-related pneumonia cases in pneumonia picture in Russia in March-May 2020: Secondary bacterial pneumonia and viral co-infections
- PMID: 33110587
- PMCID: PMC7568231
- DOI: 10.7189/jogh.10.020504
SARS-CoV-2-related pneumonia cases in pneumonia picture in Russia in March-May 2020: Secondary bacterial pneumonia and viral co-infections
Abstract
Background: We are communicating the results of investigating statistics on SARS-CoV-2-related pneumonias in Russia: percentage, mortality, cases with other viral agents, cases accompanied by secondary bacterial pneumonias, age breakdown, clinical course and outcome.
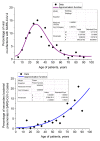
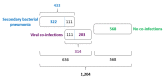
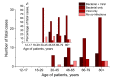
Methods: We studied two sampling sets (Set 1 and Set 2). Set 1 consisted of results of testing 3382 assays of out-patients and hospital patients (5-88 years old) with community-acquired and hospital-acquired pneumonia of yet undetermined aetiology. Set 2 contained results of 1204 assays of hospital patients (12-94 years old) with pneumonia and COVID-19 already diagnosed by molecular biological techniques in test laboratories. The results were collected in twelve Russian cities/provinces in time range 2 March - 5 May 2020. Assays were analysed for 10 bacterial, 15 viral, 2 fungal and 2 parasitic aetiological agents.
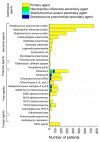
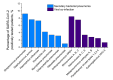
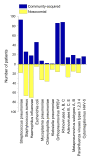
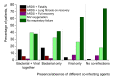
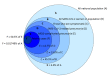
Results: In Set 1, 4.35% of total pneumonia cases were related to SARS-CoV-2, with substantially larger proportion (18.75%) of deaths of pneumonia with COVID-19 diagnosed. However, studying Set 2, we revealed that 52.82% patients in it were also positive for different typical and atypical aetiological agents usually causing pneumonia. 433 COVID-19 patients (35.96%) were tested positive for various bacterial aetiological agents, with Streptococcus pneumoniae, Staphylococcus aureus and Haemophilus influenzae infections accounting for the majority of secondary pneumonia cases.
Conclusions: SARS-CoV-2, a low-pathogenic virus itself, becomes exceptionally dangerous if secondary bacterial pneumonia attacks a COVID-19 patient as a complication. An essential part of the severest complications and mortality associated with COVID-19 in Russia in March-May 2020, may be attributed to secondary bacterial pneumonia and to a much less extent viral co-infections. The problem of hospital-acquired bacterial infection is exceptionally urgent in treating SARS-CoV-2 patients. The risk of secondary bacterial pneumonia and its further complications, should be given very serious attention in combating SARS-CoV-2.
Copyright © 2020 by the Journal of Global Health. All rights reserved.
Conflict of interest statement
Competing interests: The author completed the ICMJE Unified Competing Interest form (available upon request from the corresponding author) and declares no conflicts of interest.
Figures



Similar articles
-
Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting.Clin Microbiol Infect. 2020 Oct;26(10):1395-1399. doi: 10.1016/j.cmi.2020.06.025. Epub 2020 Jun 27. Clin Microbiol Infect. 2020. PMID: 32603803 Free PMC article.
-
Adaptation of a Russian population to SARS-CoV-2: Asymptomatic course, comorbidities, mortality, and other respiratory viruses - A reply to Fear versus Data.Int J Antimicrob Agents. 2020 Oct;56(4):106093. doi: 10.1016/j.ijantimicag.2020.106093. Epub 2020 Jul 10. Int J Antimicrob Agents. 2020. PMID: 32653618 Free PMC article.
-
Community-acquired viral respiratory infections amongst hospitalized inpatients during a COVID-19 outbreak in Singapore: co-infection and clinical outcomes.J Clin Virol. 2020 Jul;128:104436. doi: 10.1016/j.jcv.2020.104436. Epub 2020 May 19. J Clin Virol. 2020. PMID: 32447256 Free PMC article.
-
Bacterial co-infections with SARS-CoV-2.IUBMB Life. 2020 Oct;72(10):2097-2111. doi: 10.1002/iub.2356. Epub 2020 Aug 8. IUBMB Life. 2020. PMID: 32770825 Free PMC article. Review.
-
Profile of co-infections & secondary infections in COVID-19 patients at a dedicated COVID-19 facility of a tertiary care Indian hospital: Implication on antimicrobial resistance.Indian J Med Microbiol. 2021 Apr;39(2):147-153. doi: 10.1016/j.ijmmb.2020.10.014. Epub 2020 Nov 2. Indian J Med Microbiol. 2021. PMID: 33966856 Free PMC article. Review.
Cited by
-
Investigation of respiratory tract coinfections in Coronavirus disease 2019 infected and suspected cases.North Clin Istanb. 2022 Oct 20;9(5):421-428. doi: 10.14744/nci.2022.82608. eCollection 2022. North Clin Istanb. 2022. PMID: 36447585 Free PMC article.
-
8806 Russian patients demonstrate T cell count as better marker of COVID-19 clinical course severity than SARS-CoV-2 viral load.Sci Rep. 2021 May 3;11(1):9440. doi: 10.1038/s41598-021-88714-6. Sci Rep. 2021. PMID: 33941816 Free PMC article.
-
Viral Coinfection among COVID-19 Patient Groups: An Update Systematic Review and Meta-Analysis.Biomed Res Int. 2021 Sep 3;2021:5313832. doi: 10.1155/2021/5313832. eCollection 2021. Biomed Res Int. 2021. PMID: 34485513 Free PMC article.
-
Clinico-Epidemio-Microbiological Exploratory Review Among COVID-19 Patients with Secondary Infection in Central India.Infect Drug Resist. 2022 Apr 8;15:1667-1676. doi: 10.2147/IDR.S355742. eCollection 2022. Infect Drug Resist. 2022. PMID: 35422635 Free PMC article.
-
Key points of technical review for the registration of SARS-CoV-2 antigen/antibody tests.Bioanalysis. 2021 Jan;13(2):77-88. doi: 10.4155/bio-2020-0219. Epub 2021 Jan 11. Bioanalysis. 2021. PMID: 33427483 Free PMC article.
References
-
- Andrejak C, Blanc FX, Costes F, Crestani B, Perez T, Philippe B, et al. Guide pour le suivi respiratoire des patients ayant présenté une pneumonie à SARS-CoV-2. Propositions de prise en charge élaborées par la Société de Pneumologie de Langue Française. Version du 10 mai 2020. Rev Mal Respir. 2020. Epub ahead of print. 10.1016/j.rmr.2020.05.001 - DOI - PMC - PubMed
-
- Peng S, Huang L, Zhao B, Zhou S, Braithwaite I, Zhang N, et al. Clinical course of coronavirus disease 2019 in 11 patients after thoracic surgery and challenges in diagnosis. J Thorac Cardiovasc Surg. 2020. Apr 10. pii: S0022-5223(20)30859-X. Epub ahead of print. 10.1016/j.jtcvs.2020.04.005 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
