Prevalence of germline BRCA mutations in HER2-negative metastatic breast cancer: global results from the real-world, observational BREAKOUT study
- PMID: 33109210
- PMCID: PMC7590609
- DOI: 10.1186/s13058-020-01349-9
Prevalence of germline BRCA mutations in HER2-negative metastatic breast cancer: global results from the real-world, observational BREAKOUT study
Abstract
Background: The global observational BREAKOUT study investigated germline BRCA mutation (gBRCAm) prevalence in a population of patients with human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer (MBC).
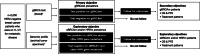
Methods: Eligible patients had initiated first-line cytotoxic chemotherapy for HER2-negative MBC within 90 days prior to enrollment. Hormone receptor (HR)-positive patients had experienced disease progression on or after prior endocrine therapy, or endocrine therapy was considered unsuitable. gBRCAm status was determined using baseline blood samples or prior germline test results. For patients with a negative gBRCAm test, archival tissue was tested for somatic BRCAm and homologous recombination repair mutations (HRRm). Details of first-line cytotoxic chemotherapy were also collected.
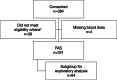
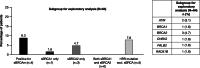
Results: Between March 2017 and April 2018, 384 patients from 14 countries were screened and consented to study enrollment; 341 patients were included in the full analysis set (median [range] age at enrollment: 56 [25-89] years; 256 (75.3%) postmenopausal). Overall, 33 patients (9.7%) had a gBRCAm (16 [4.7%] in gBRCA1 only, 12 [3.5%] in gBRCA2 only, and 5 [1.5%] in both gBRCA1 and gBRCA2). gBRCAm prevalence was similar in HR-positive and HR-negative patients. gBRCAm prevalence was 9.0% in European patients and 10.6% in Asian patients and was higher in patients aged ≤ 50 years at initial breast cancer (BC) diagnosis (12.9%) than patients aged > 50 years (5.4%). In patients with any risk factor for having a gBRCAm (family history of BC and/or ovarian cancer, aged ≤ 50 years at initial BC diagnosis, or triple-negative BC), prevalence was 10.4%, versus 5.8% in patients without these risk factors. HRRm prevalence was 14.1% (n = 9/64) in patients with germline BRCA wildtype.
Conclusions: Patient demographic and disease characteristics supported the association of a gBRCAm with younger age at initial BC diagnosis and family history of BC and/or ovarian cancer. gBRCAm prevalence in this cohort, not selected on the basis of risk factors for gBRCAm, was slightly higher than previous results suggested. gBRCAm prevalence among patients without a traditional risk factor for harboring a gBRCAm (5.8%) supports current guideline recommendations of routine gBRCAm testing in HER2-negative MBC, as these patients may benefit from poly(ADP-ribose) polymerase (PARP) inhibitor therapy.
Trial registration: NCT03078036 .
Keywords: BRCA; Breast cancer susceptibility genes; Observational; Prevalence.
Conflict of interest statement
Dr. Joyce O’Shaughnessy has received honoraria for consulting and advisory boards from AbbVie, Agendia, Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene Corporation, Eisai, Eli Lilly, Genentech, Genomic Health, GRAIL, Heron Therapeutics, Immunomedics, Ipsen Biopharmaceuticals, Jounce Therapeutics, Merck, Myriad, Novartis, Odonate Therapeutics, Pfizer, Puma Biotechnology, Roche, Seattle Genetics, and Syndax Pharmaceuticals.
Dr. Christine Brezden-Masley has received honoraria for consulting and advisory boards from Amgen, AstraZeneca, Eli Lilly, Genomic Health, Merck, Myriad, Novartis, Pfizer, Roche, and Taiho. She has received travel grants from Amgen, AstraZeneca, and Roche.
Dr. Marina Cazzaniga reports no competing interests.
Dr. Tapashi Dalvi, Dr. Graham Walker, and Dr. James Bennett are employees or contracted employees of AstraZeneca and may own stock.
Dr. Shozo Ohsumi has received honoraria for lecture fees from AstraZeneca.
Figures
Similar articles
-
BRCA-mutated breast cancer: the unmet need, challenges and therapeutic benefits of genetic testing.Br J Cancer. 2024 Nov;131(9):1400-1414. doi: 10.1038/s41416-024-02827-z. Epub 2024 Aug 30. Br J Cancer. 2024. PMID: 39215191 Free PMC article. Review.
-
Prevalence of mutations in BRCA and homologous recombination repair genes and real-world standard of care of Asian patients with HER2-negative metastatic breast cancer starting first-line systemic cytotoxic chemotherapy: subgroup analysis of the global BREAKOUT study.Breast Cancer. 2022 Jan;29(1):92-102. doi: 10.1007/s12282-021-01283-4. Epub 2021 Aug 31. Breast Cancer. 2022. PMID: 34467476 Free PMC article.
-
Clinical effectiveness of olaparib monotherapy in germline BRCA-mutated, HER2-negative metastatic breast cancer in a real-world setting: phase IIIb LUCY interim analysis.Eur J Cancer. 2021 Jul;152:68-77. doi: 10.1016/j.ejca.2021.03.029. Epub 2021 Jun 1. Eur J Cancer. 2021. PMID: 34087573 Clinical Trial.
-
Clinical Outcomes, Treatment Patterns, and Health Resource Utilization Among Metastatic Breast Cancer Patients with Germline BRCA1/2 Mutation: A Real-World Retrospective Study.Adv Ther. 2019 Mar;36(3):708-720. doi: 10.1007/s12325-018-0867-x. Epub 2019 Jan 17. Adv Ther. 2019. PMID: 30656571
-
The role of novel poly (ADP-ribose) inhibitors in the treatment of locally advanced and metastatic Her-2/neu negative breast cancer with inherited germline BRCA1/2 mutations. A review of the literature.J Med Life. 2021 Jan-Mar;14(1):17-20. doi: 10.25122/jml-2020-0132. J Med Life. 2021. PMID: 33767780 Free PMC article. Review.
Cited by
-
BRCA-mutated breast cancer: the unmet need, challenges and therapeutic benefits of genetic testing.Br J Cancer. 2024 Nov;131(9):1400-1414. doi: 10.1038/s41416-024-02827-z. Epub 2024 Aug 30. Br J Cancer. 2024. PMID: 39215191 Free PMC article. Review.
-
Prognostic and Predictive Biomarkers in Familial Breast Cancer.Cancers (Basel). 2023 Feb 20;15(4):1346. doi: 10.3390/cancers15041346. Cancers (Basel). 2023. PMID: 36831687 Free PMC article. Review.
-
Precision medicine in the real-world setting. Clinical activity of talazoparib in a woman with hormone receptor-positive HER2-negative metastatic breast cancer with pathogenic mutation in somatic BRCA2.Ecancermedicalscience. 2022 Sep 26;16:1448. doi: 10.3332/ecancer.2022.1448. eCollection 2022. Ecancermedicalscience. 2022. PMID: 36405946 Free PMC article.
-
Breast Cancer and Genetic BRCA1/2 Testing in Routine Clinical Practice: Why, When and For Whom?Geburtshilfe Frauenheilkd. 2023 Mar 9;83(3):310-320. doi: 10.1055/a-1929-2629. eCollection 2023 Mar. Geburtshilfe Frauenheilkd. 2023. PMID: 36908286 Free PMC article.
-
Combination of Small Extracellular Vesicle-Derived Annexin A2 Protein and mRNA as a Potential Predictive Biomarker for Chemotherapy Responsiveness in Aggressive Triple-Negative Breast Cancer.Cancers (Basel). 2022 Dec 29;15(1):212. doi: 10.3390/cancers15010212. Cancers (Basel). 2022. PMID: 36612209 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

