Subcellular Location of Tirapazamine Reduction Dramatically Affects Aerobic but Not Anoxic Cytotoxicity
- PMID: 33105798
- PMCID: PMC7660101
- DOI: 10.3390/molecules25214888
Subcellular Location of Tirapazamine Reduction Dramatically Affects Aerobic but Not Anoxic Cytotoxicity
Abstract
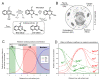
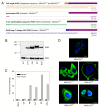
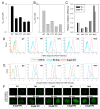
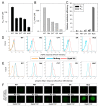
Hypoxia is an adverse prognostic feature of solid cancers that may be overcome with hypoxia-activated prodrugs (HAPs). Tirapazamine (TPZ) is a HAP which has undergone extensive clinical evaluation in this context and stimulated development of optimized analogues. However the subcellular localization of the oxidoreductases responsible for mediating TPZ-dependent DNA damage remains unclear. Some studies conclude only nuclear-localized oxidoreductases can give rise to radical-mediated DNA damage and thus cytotoxicity, whereas others identify a broader role for endoplasmic reticulum and cytosolic oxidoreductases, indicating the subcellular location of TPZ radical formation is not a critical requirement for DNA damage. To explore this question in intact cells we engineered MDA-231 breast cancer cells to express the TPZ reductase human NADPH: cytochrome P450 oxidoreductase (POR) harboring various subcellular localization sequences to guide this flavoenzyme to the nucleus, endoplasmic reticulum, cytosol or inner surface of the plasma membrane. We show that all POR variants are functional, with differences in rates of metabolism reflecting enzyme expression levels rather than intracellular TPZ concentration gradients. Under anoxic conditions, POR expression in all subcellular compartments increased the sensitivity of the cells to TPZ, but with a fall in cytotoxicity per unit of metabolism (termed 'metabolic efficiency') when POR is expressed further from the nucleus. However, under aerobic conditions a much larger increase in cytotoxicity was observed when POR was directed to the nucleus, indicating very high metabolic efficiency. Consequently, nuclear metabolism results in collapse of hypoxic selectivity of TPZ, which was further magnified to the point of reversing O2 dependence (oxic > hypoxic sensitivity) by employing a DNA-affinic TPZ analogue. This aerobic hypersensitivity phenotype was partially rescued by cellular copper depletion, suggesting the possible involvement of Fenton-like chemistry in generating short-range effects mediated by the hydroxyl radical. In addition, the data suggest that under aerobic conditions reoxidation strictly limits the TPZ radical diffusion range resulting in site-specific cytotoxicity. Collectively these novel findings challenge the purported role of intra-nuclear reductases in orchestrating the hypoxia selectivity of TPZ.
Keywords: DNA damage-response; DNA-targeted cytotoxin; cell membrane; cell nucleus; cytochrome P450 oxidoreductase; endoplasmic reticulum; hypoxia-activated prodrug; tirapazamine.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Adaptation of human tumor cells to tirapazamine under aerobic conditions: implications of increased antioxidant enzyme activity to mechanism of aerobic cytotoxicity.Biochem Pharmacol. 1997 Jul 15;54(2):249-57. doi: 10.1016/s0006-2952(97)00171-8. Biochem Pharmacol. 1997. PMID: 9271329
-
Metabolism of tirapazamine by multiple reductases in the nucleus.Biochem Pharmacol. 2001 Nov 1;62(9):1201-9. doi: 10.1016/s0006-2952(01)00784-5. Biochem Pharmacol. 2001. PMID: 11705453
-
NADPH:cytochrome c (P450) reductase activates tirapazamine (SR4233) to restore hypoxic and oxic cytotoxicity in an aerobic resistant derivative of the A549 lung cancer cell line.Br J Cancer. 2000 Feb;82(3):651-6. doi: 10.1054/bjoc.1999.0977. Br J Cancer. 2000. PMID: 10682679 Free PMC article.
-
Enzymology of tirapazamine metabolism: a review.Anticancer Drug Des. 1998 Sep;13(6):541-73. Anticancer Drug Des. 1998. PMID: 9755718 Review.
-
Antitumour prodrug development using cytochrome P450 (CYP) mediated activation.Anticancer Drug Des. 1999 Dec;14(6):473-86. Anticancer Drug Des. 1999. PMID: 10834269 Review.
Cited by
-
Redox Proteomic Profile of Tirapazamine-Resistant Murine Hepatoma Cells.Int J Mol Sci. 2023 Apr 6;24(7):6863. doi: 10.3390/ijms24076863. Int J Mol Sci. 2023. PMID: 37047836 Free PMC article.
-
Tissue Pharmacokinetic Properties and Bystander Potential of Hypoxia-Activated Prodrug CP-506 by Agent-Based Modelling.Front Pharmacol. 2022 Feb 8;13:803602. doi: 10.3389/fphar.2022.803602. eCollection 2022. Front Pharmacol. 2022. PMID: 35211015 Free PMC article.
-
Characterizing Endocrine Status, Tumor Hypoxia and Immunogenicity for Therapy Success in Epithelial Ovarian Cancer.Front Endocrinol (Lausanne). 2021 Nov 17;12:772349. doi: 10.3389/fendo.2021.772349. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34867818 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

