Anticolorectal Cancer Effects of AUCAN: Effects to Suppress Proliferation, Metastasis, and Invasion of Tumor Cells
- PMID: 33102601
- PMCID: PMC7569457
- DOI: 10.1155/2020/9786428
Anticolorectal Cancer Effects of AUCAN: Effects to Suppress Proliferation, Metastasis, and Invasion of Tumor Cells
Abstract
Background: Colorectal cancer (CRC) is an underlying deadly malignancy with poor prognosis, lacking effective therapies currently available to improve the prognosis. C18H17NO6 (AUCAN), a kind of dibenzofuran extracted from a special plant in Yunnan Province (China), is identified as a natural anticancer agent exerting strong inhibitory activities on various cancers. Our study was committed to investigating the potency of AUCAN against colorectal cancers and further exploring the potential mechanisms via proteomic analysis.
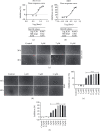
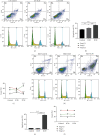
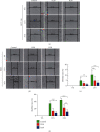
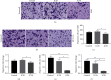
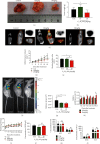
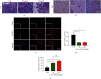
Methods: Cell Counting Kit-8 assay and immunofluorescence staining were used to investigate the effect of AUCAN on the viability and proliferation of HCT-116 cells and RKO cells. The apoptosis of HCT-116 and RKO cells after AUCAN administration was determined by the flow cytometry test. The effects of AUCAN on invasion and migration of tumor cells were investigated by the colony formation assay, wound healing test, and Transwell invasion test. Meanwhile, the energy metabolism and growth of tumor tissues after AUCAN administration with 10 mg/kg and 20 mg/kg were examined by PET-CT in vivo. The side effects of AUCAN treatment were also evaluated through blood routine and liver function examination. RKO cell proliferation and apoptosis in vivo were further determined by hematoxylin and eosin staining, TUNEL staining, and immunohistochemistry. Furthermore, the differentially expressed proteins (DEPs) involved in AUCAN treatment were determined by proteomic analysis followed by functional clustering analysis.
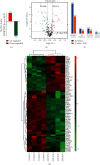
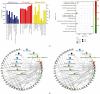
Results: The results showed that AUCAN suppressed the migratory abilities and enhanced apoptosis of HCT-116 and RKO cell lines. Meanwhile, AUCAN treatment dramatically depressed the growth and volume of colorectal tumors in nude mice and suppressed the survival of RKO cells in tumor tissues without any side effects on the blood routine and liver function. In addition, twenty-four upregulated and forty-two downregulated proteins were identified. Additionally, functional clustering analysis concealed enriched biological processes, cellular components, molecular functions, and related pathways of these proteins involved in cellular metabolic. Finally, the protein-protein interaction analysis revealed the regulatory connection among these DEPs.
Conclusions: Taken together, AUCAN exerted its significant antitumor effect without side effects in the blood routine and liver function and the underlying mechanisms were preliminarily investigated by proteomic analysis.
Copyright © 2020 Liu-Lin Xiong et al.
Conflict of interest statement
The authors claim no competing financial interests.
Figures


Similar articles
-
Anti-colorectal cancer effects of scutellarin revealed by genomic and proteomic analysis.Chin Med. 2020 Mar 26;15:28. doi: 10.1186/s13020-020-00307-z. eCollection 2020. Chin Med. 2020. PMID: 32226478 Free PMC article.
-
Antitumor activity in colorectal cancer induced by hinokiflavone.J Gastroenterol Hepatol. 2019 Sep;34(9):1571-1580. doi: 10.1111/jgh.14581. Epub 2019 Jan 16. J Gastroenterol Hepatol. 2019. PMID: 30575109
-
Pristimerin inhibits proliferation, migration and invasion, and induces apoptosis in HCT-116 colorectal cancer cells.Biomed Pharmacother. 2016 Apr;79:112-9. doi: 10.1016/j.biopha.2016.02.003. Epub 2016 Feb 18. Biomed Pharmacother. 2016. PMID: 27044819
-
Antitumor effects of IOX1 combined with bevacizumab-induced apoptosis and immunity on colorectal cancer cells.Int Immunopharmacol. 2024 Nov 15;141:112896. doi: 10.1016/j.intimp.2024.112896. Epub 2024 Aug 14. Int Immunopharmacol. 2024. PMID: 39146782
-
Exploring the multi-targeting phytoestrogen potential of Calycosin for cancer treatment: A review.Medicine (Baltimore). 2024 May 3;103(18):e38023. doi: 10.1097/MD.0000000000038023. Medicine (Baltimore). 2024. PMID: 38701310 Free PMC article. Review.
Cited by
-
Using omics approaches to dissect the therapeutic effects of Chinese herbal medicines on gastrointestinal cancers.Front Pharmacol. 2022 Sep 23;13:884822. doi: 10.3389/fphar.2022.884822. eCollection 2022. Front Pharmacol. 2022. PMID: 36210831 Free PMC article. Review.
References
-
- Edwards B. K., Ward E., Kohler B. A., et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. doi: 10.1002/cncr.24760. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

