Neutrophil: A New Player in Metastatic Cancers
- PMID: 33101283
- PMCID: PMC7546851
- DOI: 10.3389/fimmu.2020.565165
Neutrophil: A New Player in Metastatic Cancers
Abstract
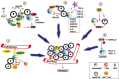
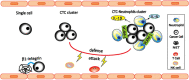
The interaction between cancer cells and immune cells is important for the cancer development. However, much attention has been given to T cells and macrophages. Being the most abundant leukocytes in the blood, the functions of neutrophils in cancer have been underdetermined. They have long been considered an "audience" in the development of cancer. However, emerging evidence indicate that neutrophils are a heterogeneous population with plasticity, and subpopulation of neutrophils (such as low density neutrophils, polymorphonuclear-myeloid-derived suppressor cells) are actively involved in cancer growth and metastasis. Here, we review the current understanding of the role of neutrophils in cancer development, with a specific focus on their pro-metastatic functions. We also discuss the potential and challenges of neutrophils as therapeutic targets. A better understanding the role of neutrophils in cancer will discover new mechanisms of metastasis and develop new immunotherapies by targeting neutrophils.
Keywords: circulating tumor cells; immunosuppression; metastasis; neutrophils; pre-metastatic niche; tumor microenvironments.
Copyright © 2020 Wu, Ma, Tan, Zheng and Liu.
Figures


Similar articles
-
Neutrophils: important contributors to tumor progression and metastasis.Cancer Metastasis Rev. 2015 Dec;34(4):735-51. doi: 10.1007/s10555-015-9594-9. Cancer Metastasis Rev. 2015. PMID: 26361774 Review.
-
Myeloid Cells in Metastasis.Cold Spring Harb Perspect Med. 2020 May 1;10(5):a038026. doi: 10.1101/cshperspect.a038026. Cold Spring Harb Perspect Med. 2020. PMID: 31548218 Free PMC article. Review.
-
Tumor promoting capacity of polymorphonuclear myeloid-derived suppressor cells and their neutralization.Int J Cancer. 2021 Nov 1;149(9):1628-1638. doi: 10.1002/ijc.33731. Epub 2021 Jul 17. Int J Cancer. 2021. PMID: 34224592 Review.
-
Targeting and exploitation of tumor-associated neutrophils to enhance immunotherapy and drug delivery for cancer treatment.Cancer Biol Med. 2020 Feb 15;17(1):32-43. doi: 10.20892/j.issn.2095-3941.2019.0372. Cancer Biol Med. 2020. PMID: 32296575 Free PMC article. Review.
-
Circulating Tumor Cell-Neutrophil Tango along the Metastatic Process.Cancer Res. 2019 Dec 15;79(24):6067-6073. doi: 10.1158/0008-5472.CAN-19-1972. Epub 2019 Sep 16. Cancer Res. 2019. PMID: 31527091 Review.
Cited by
-
Tumour extracellular vesicles induce neutrophil extracellular traps to promote lymph node metastasis.J Extracell Vesicles. 2023 Aug;12(8):e12341. doi: 10.1002/jev2.12341. J Extracell Vesicles. 2023. PMID: 37563798 Free PMC article.
-
Novel Characterization of Myeloid-Derived Suppressor Cells in Tumor Microenvironment.Front Cell Dev Biol. 2021 Aug 30;9:698532. doi: 10.3389/fcell.2021.698532. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34527668 Free PMC article. Review.
-
c-Met Mediated Cytokine Network Promotes Brain Metastasis of Breast Cancer by Remodeling Neutrophil Activities.Cancers (Basel). 2023 May 5;15(9):2626. doi: 10.3390/cancers15092626. Cancers (Basel). 2023. PMID: 37174093 Free PMC article.
-
Chemokines orchestrate tumor cells and the microenvironment to achieve metastatic heterogeneity.Cancer Metastasis Rev. 2021 Jun;40(2):447-476. doi: 10.1007/s10555-021-09970-6. Epub 2021 May 6. Cancer Metastasis Rev. 2021. PMID: 33959849 Free PMC article. Review.
-
Modulation of T-cell function by myeloid-derived suppressor cells in hematological malignancies.Front Cell Dev Biol. 2023 Apr 5;11:1129343. doi: 10.3389/fcell.2023.1129343. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37091970 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

