Extracellular Vesicles in the Pathogenesis of Viral Infections in Humans
- PMID: 33096825
- PMCID: PMC7589806
- DOI: 10.3390/v12101200
Extracellular Vesicles in the Pathogenesis of Viral Infections in Humans
Abstract
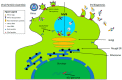
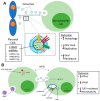
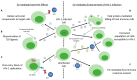
Most cells can release extracellular vesicles (EVs), membrane vesicles containing various proteins, nucleic acids, enzymes, and signaling molecules. The exchange of EVs between cells facilitates intercellular communication, amplification of cellular responses, immune response modulation, and perhaps alterations in viral pathogenicity. EVs serve a dual role in inhibiting or enhancing viral infection and pathogenesis. This review examines the current literature on EVs to explore the complex role of EVs in the enhancement, inhibition, and potential use as a nanotherapeutic against clinically relevant viruses, focusing on neurotropic viruses: Zika virus (ZIKV) and human immunodeficiency virus (HIV). Overall, this review's scope will elaborate on EV-based mechanisms, which impact viral pathogenicity, facilitate viral spread, and modulate antiviral immune responses.
Keywords: HIV; ZIKA; coronavirus; exosomes; extracellular vesicles (EVs); herpes virus; pathology; retrovirus; therapeutics; viruses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The Role of Extracellular Vesicles as Allies of HIV, HCV and SARS Viruses.Viruses. 2020 May 22;12(5):571. doi: 10.3390/v12050571. Viruses. 2020. PMID: 32456011 Free PMC article. Review.
-
The complex role of extracellular vesicles in HIV infection.BMB Rep. 2023 Jun;56(6):335-340. doi: 10.5483/BMBRep.2023-0073. BMB Rep. 2023. PMID: 37291055 Free PMC article. Review.
-
Extracellular Vesicles Are Conveyors of the NS1 Toxin during Dengue Virus and Zika Virus Infection.Viruses. 2023 Jan 27;15(2):364. doi: 10.3390/v15020364. Viruses. 2023. PMID: 36851578 Free PMC article.
-
Delivery of microRNAs by Extracellular Vesicles in Viral Infections: Could the News be Packaged?Cells. 2019 Jun 18;8(6):611. doi: 10.3390/cells8060611. Cells. 2019. PMID: 31216738 Free PMC article. Review.
-
Extracellular Vesicles in Viral Replication and Pathogenesis and Their Potential Role in Therapeutic Intervention.Viruses. 2020 Aug 13;12(8):887. doi: 10.3390/v12080887. Viruses. 2020. PMID: 32823684 Free PMC article. Review.
Cited by
-
The Use of CBD and Its Synthetic Analog HU308 in HIV-1-Infected Myeloid Cells.Pharmaceuticals (Basel). 2023 Aug 12;16(8):1147. doi: 10.3390/ph16081147. Pharmaceuticals (Basel). 2023. PMID: 37631062 Free PMC article.
-
HIV and Drug Use: A Tale of Synergy in Pulmonary Vascular Disease Development.Compr Physiol. 2023 Jun 26;13(3):4659-4683. doi: 10.1002/cphy.c210049. Compr Physiol. 2023. PMID: 37358518 Free PMC article.
-
Beyond Borders of the Cell: How Extracellular Vesicles Shape COVID-19 for People with Cystic Fibrosis.Int J Mol Sci. 2024 Mar 27;25(7):3713. doi: 10.3390/ijms25073713. Int J Mol Sci. 2024. PMID: 38612524 Free PMC article. Review.
-
Zika Virus-Infected Monocyte Exosomes Mediate Cell-to-Cell Viral Transmission.Cells. 2024 Jan 12;13(2):144. doi: 10.3390/cells13020144. Cells. 2024. PMID: 38247836 Free PMC article.
-
PPRV-Induced Autophagy Facilitates Infectious Virus Transmission by the Exosomal Pathway.J Virol. 2022 Apr 13;96(7):e0024422. doi: 10.1128/jvi.00244-22. Epub 2022 Mar 23. J Virol. 2022. PMID: 35319226 Free PMC article.
References
-
- Johnstone R.M., Adam M., Hammond J.R., Orr L., Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J. Biol. Chem. 1987;262:9412–9420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

