Power of two: combination of therapeutic approaches involving glucose transporter (GLUT) inhibitors to combat cancer
- PMID: 33096154
- PMCID: PMC7704680
- DOI: 10.1016/j.bbcan.2020.188457
Power of two: combination of therapeutic approaches involving glucose transporter (GLUT) inhibitors to combat cancer
Abstract
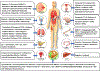
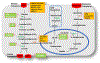
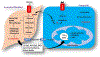
Cancer research of the Warburg effect, a hallmark metabolic alteration in tumors, focused attention on glucose metabolism whose targeting uncovered several agents with promising anticancer effects at the preclinical level. These agents' monotherapy points to their potential as adjuvant combination therapy to existing standard chemotherapy in human trials. Accordingly, several studies on combining glucose transporter (GLUT) inhibitors with chemotherapeutic agents, such as doxorubicin, paclitaxel, and cytarabine, showed synergistic or additive anticancer effects, reduced chemo-, radio-, and immuno-resistance, and reduced toxicity due to lowering the therapeutic doses required for desired chemotherapeutic effects, as compared with monotherapy. The combinations have been specifically effective in treating cancer glycolytic phenotypes, such as pancreatic and breast cancers. Even combining GLUT inhibitors with other glycolytic inhibitors and energy restriction mimetics seems worthwhile. Though combination clinical trials are in the early phase, initial results are intriguing. The various types of GLUTs, their role in cancer progression, GLUT inhibitors, and their anticancer mechanism of action have been reviewed several times. However, utilizing GLUT inhibitors as combination therapeutics has received little attention. We consider GLUT inhibitors agents that directly affect glucose transporters by binding to them or indirectly alter glucose transport by changing the transporters' expression level. This review mainly focuses on summarizing the effects of various combinations of GLUT inhibitors with other anticancer agents and providing a perspective on the current status.
Keywords: Cancer; Combination strategy; Drug synergy; GLUT inhibitors; Glucose transporters (GLUTs).
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Figures
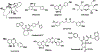

Similar articles
-
Targeting Key Transporters in Tumor Glycolysis as a Novel Anticancer Strategy.Curr Top Med Chem. 2018;18(6):454-466. doi: 10.2174/1568026618666180523105234. Curr Top Med Chem. 2018. PMID: 29788889 Review.
-
Glucose Transporter Regulation in Cancer: A Profile and the Loops.Crit Rev Eukaryot Gene Expr. 2016;26(3):223-38. doi: 10.1615/CritRevEukaryotGeneExpr.2016016531. Crit Rev Eukaryot Gene Expr. 2016. PMID: 27650986 Review.
-
Glycolysis Inhibitors for Anticancer Therapy: A Review of Recent Patents.Recent Pat Anticancer Drug Discov. 2016;11(3):297-308. doi: 10.2174/1574892811666160415160104. Recent Pat Anticancer Drug Discov. 2016. PMID: 27087655 Review.
-
Hypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: novel pathways and targets for anticancer therapeutics.Chemotherapy. 2007;53(4):233-56. doi: 10.1159/000104457. Epub 2007 Jun 25. Chemotherapy. 2007. PMID: 17595539 Review.
-
Identification of a Small-Molecule Glucose Transporter Inhibitor, Glutipyran, That Inhibits Cancer Cell Growth.ACS Chem Biol. 2021 Aug 20;16(8):1576-1586. doi: 10.1021/acschembio.1c00480. Epub 2021 Jul 23. ACS Chem Biol. 2021. PMID: 34296611
Cited by
-
Targeting Glucose Metabolism Enzymes in Cancer Treatment: Current and Emerging Strategies.Cancers (Basel). 2022 Sep 21;14(19):4568. doi: 10.3390/cancers14194568. Cancers (Basel). 2022. PMID: 36230492 Free PMC article. Review.
-
GLUT5: structure, functions, diseases and potential applications.Acta Biochim Biophys Sin (Shanghai). 2023 Oct 25;55(10):1519-1538. doi: 10.3724/abbs.2023158. Acta Biochim Biophys Sin (Shanghai). 2023. PMID: 37674366 Free PMC article. Review.
-
Modulating Glycolysis to Improve Cancer Therapy.Int J Mol Sci. 2023 Jan 30;24(3):2606. doi: 10.3390/ijms24032606. Int J Mol Sci. 2023. PMID: 36768924 Free PMC article. Review.
-
Tumor microenvironment-activated cancer cell membrane-liposome hybrid nanoparticle-mediated synergistic metabolic therapy and chemotherapy for non-small cell lung cancer.J Nanobiotechnology. 2021 Oct 24;19(1):339. doi: 10.1186/s12951-021-01085-y. J Nanobiotechnology. 2021. PMID: 34689761 Free PMC article.
-
New Insights in ATP Synthesis as Therapeutic Target in Cancer and Angiogenic Ocular Diseases.J Histochem Cytochem. 2024 May;72(5):329-352. doi: 10.1369/00221554241249515. Epub 2024 May 11. J Histochem Cytochem. 2024. PMID: 38733294 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

