Predicting future rates of tau accumulation on PET
- PMID: 33094327
- PMCID: PMC7586089
- DOI: 10.1093/brain/awaa248
Predicting future rates of tau accumulation on PET
Abstract
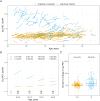
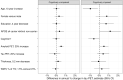
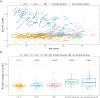
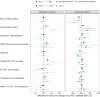
Clinical trials with anti-tau drugs will need to target individuals at risk of accumulating tau. Our objective was to identify variables available in a research setting that predict future rates of tau PET accumulation separately among individuals who were either cognitively unimpaired or cognitively impaired. All 337 participants had: a baseline study visit with MRI, amyloid PET, and tau PET exams, at least one follow-up tau PET exam; and met clinical criteria for membership in one of two clinical diagnostic groups: cognitively unimpaired (n = 203); or cognitively impaired (n = 134, a combined group of participants with either mild cognitive impairment or dementia with Alzheimer's clinical syndrome). Our primary analyses were in these two clinical groups; however, we also evaluated subgroups dividing the unimpaired group by normal/abnormal amyloid PET and the impaired group by clinical phenotype (mild cognitive impairment, amnestic dementia, and non-amnestic dementia). Linear mixed effects models were used to estimate associations between age, sex, education, APOE genotype, amyloid and tau PET standardized uptake value ratio (SUVR), cognitive performance, cortical thickness, and white matter hyperintensity volume at baseline, and the rate of subsequent tau PET accumulation. Log-transformed tau PET SUVR was used as the response and rates were summarized as annual per cent change. A temporal lobe tau PET meta-region of interest was used. In the cognitively unimpaired group, only higher baseline amyloid PET was a significant independent predictor of higher tau accumulation rates (P < 0.001). Higher rates of tau accumulation were associated with faster rates of cognitive decline in the cognitively unimpaired subgroup with abnormal amyloid PET (P = 0.03), but among the subgroup with normal amyloid PET. In the cognitively impaired group, younger age (P = 0.02), higher baseline amyloid PET (P = 0.05), APOE ε4 (P = 0.05), and better cognitive performance (P = 0.05) were significant independent predictors of higher tau accumulation rates. Among impaired individuals, faster cognitive decline was associated with faster rates of tau accumulation (P = 0.01). While we examined many possible predictor variables, our results indicate that screening of unimpaired individuals for potential inclusion in anti-tau trials may be straightforward because the only independent predictor of high tau rates was amyloidosis. In cognitively impaired individuals, imaging and clinical variables consistent with early onset Alzheimer's disease phenotype were associated with higher rates of tau PET accumulation suggesting this may be a highly advantageous group in which to conduct proof-of-concept clinical trials that target tau-related mechanisms. The nature of the dementia phenotype (amnestic versus non-amnestic) did not affect this conclusion.
Keywords: Alzheimer’s disease; Alzheimer’s disease clinical trials; serial tau PET; tau; tau PET.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures
Similar articles
-
Longitudinal tau PET in ageing and Alzheimer's disease.Brain. 2018 May 1;141(5):1517-1528. doi: 10.1093/brain/awy059. Brain. 2018. PMID: 29538647 Free PMC article.
-
The accumulation rate of tau aggregates is higher in females and younger amyloid-positive subjects.Brain. 2020 Dec 1;143(12):3805-3815. doi: 10.1093/brain/awaa327. Brain. 2020. PMID: 33439987 Free PMC article.
-
Amyloid and Tau Prediction of Cognitive and Functional Decline in Unimpaired Older Individuals: Longitudinal Data from the A4 and LEARN Studies.J Prev Alzheimers Dis. 2024;11(4):802-813. doi: 10.14283/jpad.2024.122. J Prev Alzheimers Dis. 2024. PMID: 39044488 Free PMC article. Clinical Trial.
-
White matter hyperintensities are higher among early-onset Alzheimer's disease participants than their cognitively normal and early-onset nonAD peers: Longitudinal Early-onset Alzheimer's Disease Study (LEADS).Alzheimers Dement. 2023 Nov;19 Suppl 9(Suppl 9):S89-S97. doi: 10.1002/alz.13402. Epub 2023 Jul 25. Alzheimers Dement. 2023. PMID: 37491599 Free PMC article. Review.
-
Sleep disorders in Alzheimer's disease: the predictive roles and potential mechanisms.Neural Regen Res. 2021 Oct;16(10):1965-1972. doi: 10.4103/1673-5374.308071. Neural Regen Res. 2021. PMID: 33642368 Free PMC article. Review.
Cited by
-
Lipid Profiling of Alzheimer's Disease Brain Highlights Enrichment in Glycerol(phospho)lipid, and Sphingolipid Metabolism.Cells. 2021 Sep 29;10(10):2591. doi: 10.3390/cells10102591. Cells. 2021. PMID: 34685570 Free PMC article.
-
Verbal memory formation across PET-based Braak stages of tau accumulation in Alzheimer's disease.Brain Commun. 2023 May 18;5(3):fcad146. doi: 10.1093/braincomms/fcad146. eCollection 2023. Brain Commun. 2023. PMID: 37252014 Free PMC article.
-
Recent Advances in Imaging of Preclinical, Sporadic, and Autosomal Dominant Alzheimer's Disease.Neurotherapeutics. 2021 Apr;18(2):709-727. doi: 10.1007/s13311-021-01026-5. Epub 2021 Mar 29. Neurotherapeutics. 2021. PMID: 33782864 Free PMC article. Review.
-
Dynamic network model reveals distinct tau spreading patterns in early- and late-onset Alzheimer disease.Alzheimers Res Ther. 2022 Sep 2;14(1):121. doi: 10.1186/s13195-022-01061-0. Alzheimers Res Ther. 2022. PMID: 36056405 Free PMC article.
-
Deep learning identifies brain structures that predict cognition and explain heterogeneity in cognitive aging.Neuroimage. 2022 May 1;251:119020. doi: 10.1016/j.neuroimage.2022.119020. Epub 2022 Feb 20. Neuroimage. 2022. PMID: 35196565 Free PMC article.
References
-
- Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, et al.Gender differences in the incidence of AD and vascular dementia: the EURODEM Studies. EURODEM Incidence Research Group. Neurology 1999; 53: 1992–7. - PubMed
-
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT.. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology 1992; 42: 631–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

