Δ42PD1-TLR4 Augments γδ-T Cell Activation of the Transitional Memory Subset of CD4+ T Cells
- PMID: 33089108
- PMCID: PMC7567942
- DOI: 10.1016/j.isci.2020.101620
Δ42PD1-TLR4 Augments γδ-T Cell Activation of the Transitional Memory Subset of CD4+ T Cells
Abstract
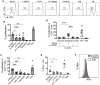
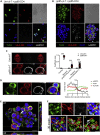
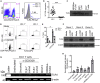
TLR ligands can contribute to T cell immune responses by indirectly stimulating antigen presentation and cytokines and directly serving as co-stimulatory signals. We have previously reported that the human endogenous surface protein, Δ42PD1, is expressed primarily on (Vγ9)Vδ2 cells and can interact with TLR4. Since Vδ2 cells possess antigen presentation capacity, we sought to further characterize if the Δ42PD1-TLR4 interaction has a role in stimulating T cell responses. In this study, we found that stimulation of Vδ2 cells not only upregulated Δ42PD1 expression but also increased MHC class II molecules necessary for the antigen presentation. In a mixed leukocyte reaction assay, upregulation of Δ42PD1 on Vδ2 cells elevated subsequent T cell proliferation. Furthermore, the interaction between Δ42PD1-TLR4 augments Vδ2 cell stimulation of autologous CMV pp65-or TT-specific CD4+ T cell proliferation and IFN-γ responses, which was specifically and significantly reduced by blocking the Δ42PD1-TLR4 interaction. Furthermore, confocal microscopy analysis confirmed the interaction between Δ42PD1+HLA-DR+Vδ2 cells and TLR4+CD4 T cells. Interestingly, the subset of CD4+ T cells expressing TLR4 appears to be PD-1+ CD45RO+CD45RA+ transitional memory T cells and responded to Δ42PD1+HLA-DR+Vδ2 cells. Overall, this study demonstrated an important biological role of Δ42PD1 protein exhibited by Vδ2 antigen-presenting cells in augmenting T cell activation through TLR4, which may serve as an additional co-stimulatory signal.
Keywords: Cell Biology; Immunology.
© 2020 The Authors.
Conflict of interest statement
The antibodies against Δ42PD1 is described in the US patent no. 10,047,137B2 (A.K.L.C. and Z.C.).
Figures

Similar articles
-
Imaging and analysis on the interaction between human antigen-pulsed Vδ2 T cells and antigen-specific CD4 T cells.STAR Protoc. 2021 Apr 14;2(2):100453. doi: 10.1016/j.xpro.2021.100453. eCollection 2021 Jun 18. STAR Protoc. 2021. PMID: 33937873 Free PMC article.
-
Gut-homing Δ42PD1+Vδ2 T cells promote innate mucosal damage via TLR4 during acute HIV type 1 infection.Nat Microbiol. 2017 Oct;2(10):1389-1402. doi: 10.1038/s41564-017-0006-5. Epub 2017 Aug 14. Nat Microbiol. 2017. PMID: 28808299
-
Interferon-γ upregulates Δ42PD1 expression on human monocytes via the PI3K/AKT pathway.Immunobiology. 2019 May;224(3):388-396. doi: 10.1016/j.imbio.2019.02.009. Epub 2019 Feb 21. Immunobiology. 2019. PMID: 30846331
-
Direct and Indirect Effects of Cytomegalovirus-Induced γδ T Cells after Kidney Transplantation.Front Immunol. 2015 Jan 21;6:3. doi: 10.3389/fimmu.2015.00003. eCollection 2015. Front Immunol. 2015. PMID: 25653652 Free PMC article. Review.
-
Biology of gammadelta T cells in tuberculosis and malaria.Curr Mol Med. 2001 Sep;1(4):437-46. doi: 10.2174/1566524013363627. Curr Mol Med. 2001. PMID: 11899088 Review.
Cited by
-
Celastrol Downmodulates Alpha-Synuclein-Specific T Cell Responses by Mediating Antigen Trafficking in Dendritic Cells.Front Immunol. 2022 Mar 2;13:833515. doi: 10.3389/fimmu.2022.833515. eCollection 2022. Front Immunol. 2022. PMID: 35309340 Free PMC article.
-
A Δ42PD1 fusion-expressing DNA vaccine elicits enhanced adaptive immune response to HIV-1: the key role of TLR4.Virol J. 2022 Nov 1;19(1):174. doi: 10.1186/s12985-022-01909-9. Virol J. 2022. PMID: 36320043 Free PMC article.
-
Imaging and analysis on the interaction between human antigen-pulsed Vδ2 T cells and antigen-specific CD4 T cells.STAR Protoc. 2021 Apr 14;2(2):100453. doi: 10.1016/j.xpro.2021.100453. eCollection 2021 Jun 18. STAR Protoc. 2021. PMID: 33937873 Free PMC article.
References
-
- Apetoh L., Ghiringhelli F., Tesniere A., Obeid M., Ortiz C., Criollo A., Mignot G., Maiuri M.C., Ullrich E., Saulnier P. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007;13:1050–1059. - PubMed
-
- Baars P.A., Maurice M.M., Rep M., Hooibrink B., Van Lier R.A. Heterogeneity of the circulating human CD4+ T cell population. Further evidence that the CD4+CD45RA-CD27- T cell subset contains specialized primed T cells. J. Immunol. 1995;154:17–25. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

