A Rapid Caspase-11 Response Induced by IFN γ Priming Is Independent of Guanylate Binding Proteins
- PMID: 33089101
- PMCID: PMC7566093
- DOI: 10.1016/j.isci.2020.101612
A Rapid Caspase-11 Response Induced by IFN γ Priming Is Independent of Guanylate Binding Proteins
Abstract
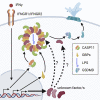
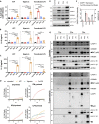
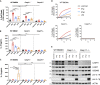
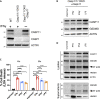
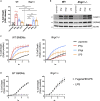
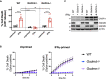
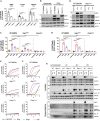
In mammalian cells, inflammatory caspases detect Gram-negative bacterial invasion by binding lipopolysaccharides (LPS). Murine caspase-11 binds cytosolic LPS, stimulates pyroptotic cell death, and drives sepsis pathogenesis. Extracellular priming factors enhance caspase-11-dependent pyroptosis. Herein we compare priming agents and demonstrate that IFNγ priming elicits the most rapid and amplified macrophage response to cytosolic LPS. Previous studies indicate that IFN-induced expression of caspase-11 and guanylate binding proteins (GBPs) are causal events explaining the effects of priming on cytosolic LPS sensing. We demonstrate that these events cannot fully account for the increased response triggered by IFNγ treatment. Indeed, IFNγ priming elicits higher pyroptosis levels in response to cytosolic LPS when macrophages stably express caspase-11. In macrophages lacking GBPs encoded on chromosome 3, IFNγ priming enhanced pyroptosis in response to cytosolic LPS as compared with other priming agents. These results suggest an unknown regulator of caspase-11-dependent pyroptosis exists, whose activity is upregulated by IFNγ.
Keywords: Cell Biology; Immunology.
© 2020 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Caspase-11 non-canonical inflammasome: a critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses.Immunology. 2017 Oct;152(2):207-217. doi: 10.1111/imm.12787. Epub 2017 Jul 31. Immunology. 2017. PMID: 28695629 Free PMC article. Review.
-
Constitutive Interferon Maintains GBP Expression Required for Release of Bacterial Components Upstream of Pyroptosis and Anti-DNA Responses.Cell Rep. 2018 Jul 3;24(1):155-168.e5. doi: 10.1016/j.celrep.2018.06.012. Cell Rep. 2018. PMID: 29972777 Free PMC article.
-
Intracellular Lipopolysaccharide Sensing as a Potential Therapeutic Target for Sepsis.Trends Pharmacol Sci. 2019 Mar;40(3):187-197. doi: 10.1016/j.tips.2019.01.001. Epub 2019 Jan 25. Trends Pharmacol Sci. 2019. PMID: 30691865 Review.
-
Sphingosine-1-phosphate Receptor 2 Signaling Promotes Caspase-11-dependent Macrophage Pyroptosis and Worsens Escherichia coli Sepsis Outcome.Anesthesiology. 2018 Aug;129(2):311-320. doi: 10.1097/ALN.0000000000002196. Anesthesiology. 2018. PMID: 29620575
-
Inhibition of Alveolar Macrophage Pyroptosis Reduces Lipopolysaccharide-induced Acute Lung Injury in Mice.Chin Med J (Engl). 2015 Oct 5;128(19):2638-45. doi: 10.4103/0366-6999.166039. Chin Med J (Engl). 2015. PMID: 26415803 Free PMC article.
Cited by
-
Inflammasome Activation in Pulmonary Arterial Hypertension.Front Med (Lausanne). 2022 Jan 13;8:826557. doi: 10.3389/fmed.2021.826557. eCollection 2021. Front Med (Lausanne). 2022. PMID: 35096915 Free PMC article. Review.
-
Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes.bioRxiv [Preprint]. 2020 Nov 13:2020.10.29.361048. doi: 10.1101/2020.10.29.361048. bioRxiv. 2020. Update in: Cell. 2021 Jan 7;184(1):149-168.e17. doi: 10.1016/j.cell.2020.11.025. PMID: 33140051 Free PMC article. Updated. Preprint.
-
Shigella OspC3 suppresses murine cytosolic LPS sensing.iScience. 2021 Jul 28;24(8):102910. doi: 10.1016/j.isci.2021.102910. eCollection 2021 Aug 20. iScience. 2021. PMID: 34409271 Free PMC article.
-
NLRP inflammasomes in health and disease.Mol Biomed. 2024 Apr 22;5(1):14. doi: 10.1186/s43556-024-00179-x. Mol Biomed. 2024. PMID: 38644450 Free PMC article. Review.
-
Lipid-protein interactions regulating the canonical and the non-canonical NLRP3 inflammasome.Prog Lipid Res. 2022 Jul;87:101182. doi: 10.1016/j.plipres.2022.101182. Epub 2022 Jul 25. Prog Lipid Res. 2022. PMID: 35901922 Free PMC article. Review.
References
-
- Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

