Drug-induced cholestasis assay in primary hepatocytes
- PMID: 33088729
- PMCID: PMC7559231
- DOI: 10.1016/j.mex.2020.101080
Drug-induced cholestasis assay in primary hepatocytes
Abstract
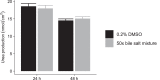
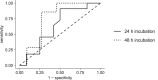
Drug-induced cholestasis (DIC) is a major cause of clinical failure of drug candidates. Numerous patients worldwide are affected when exposed to marketed drugs exhibiting a DIC signature. Prospective identification of DIC during early compound development remains challenging. Here we describe the optimized in vitro procedure for early assessment and prediction of an increased DIC risk. Our method is based on three principles:•Exposure of primary human hepatocyte cultures to test compounds in the absence and presence of a physiologically relevant mixture of endogenous bile salts.•Rapid and quantitative assessment of the influence of concomitant bile salt exposure on hepatocyte functionality and integrity after 24 h or 48 h of incubation.•Translation of the in vitro result, expressed as a DIC index (DICI) value, into an in vivo safety margin.Using our historical control data, a new (data driven) DICI cut-off value of 0.78 was established for discerning cholestatic and non-cholestatic compounds. Our DIC assay protocol was further improved by now relying on the principle of the no observable adverse effect level (NOAEL) for determining the highest test compound concentration corresponding to a DICI ≥ 0.78. Predicted safety margin values were subsequently calculated for compounds displaying hepatotoxic and/or cholestatic effects in patients, thus enabling evaluation of the performance of our DIC assay. Of note, this assay can be extended to explore the role of drug metabolites in precipitating DIC.
Keywords: Drug candidate selection; Drug safety evaluation; Drug-induced cholestasis; Drug-induced liver injury; In vitro toxicity; Primary hepatocytes.
© 2020 The Author(s). Published by Elsevier B.V.
Figures


Similar articles
-
Hepatocyte-based in vitro model for assessment of drug-induced cholestasis.Toxicol Appl Pharmacol. 2014 Jan 1;274(1):124-36. doi: 10.1016/j.taap.2013.10.032. Epub 2013 Nov 7. Toxicol Appl Pharmacol. 2014. PMID: 24211272
-
Drug-induced cholestasis risk assessment in sandwich-cultured human hepatocytes.Toxicol In Vitro. 2016 Aug;34:179-186. doi: 10.1016/j.tiv.2016.03.008. Epub 2016 Apr 2. Toxicol In Vitro. 2016. PMID: 27046439
-
Rat precision-cut liver slices predict drug-induced cholestatic injury.Arch Toxicol. 2017 Oct;91(10):3403-3413. doi: 10.1007/s00204-017-1960-7. Epub 2017 Apr 8. Arch Toxicol. 2017. PMID: 28391356 Free PMC article.
-
Current insights in the complexities underlying drug-induced cholestasis.Crit Rev Toxicol. 2019 Jul;49(6):520-548. doi: 10.1080/10408444.2019.1635081. Epub 2019 Oct 7. Crit Rev Toxicol. 2019. PMID: 31589080 Review.
-
A Change in Bile Flow: Looking Beyond Transporter Inhibition in the Development of Drug-induced Cholestasis.Curr Drug Metab. 2019;20(8):621-632. doi: 10.2174/1389200220666190709170256. Curr Drug Metab. 2019. PMID: 31288715 Free PMC article. Review.
Cited by
-
Mitochondrial GpC and CpG DNA Hypermethylation Cause Metabolic Stress-Induced Mitophagy and Cholestophagy.Int J Mol Sci. 2023 Nov 16;24(22):16412. doi: 10.3390/ijms242216412. Int J Mol Sci. 2023. PMID: 38003603 Free PMC article.
-
In Vitro Liver Toxicity Testing of Chemicals: A Pragmatic Approach.Int J Mol Sci. 2021 May 10;22(9):5038. doi: 10.3390/ijms22095038. Int J Mol Sci. 2021. PMID: 34068678 Free PMC article. Review.
-
Mouse precision-cut liver slices as an ex vivo model to study drug-induced cholestasis.Arch Toxicol. 2022 Sep;96(9):2523-2543. doi: 10.1007/s00204-022-03321-2. Epub 2022 Jun 16. Arch Toxicol. 2022. PMID: 35708773 Free PMC article.
References
-
- Morgan R.E., Trauner M., van Staden C.J., Lee P.H., Ramachandran B., Eschenberg M., Afshari C.A., Qualls C.W., Lightfoot-Dunn R., Hamadeh H.K. Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol. Sci. 2010;118:485–500. doi: 10.1093/toxsci/kfq269. - DOI - PubMed
-
- De Bruyn T., Chatterjee S., Fattah S., Keemink J., Nicolaï J., Augustijns P., Annaert P. Sandwich-cultured hepatocytes: utility for in vitro exploration of hepatobiliary drug disposition and drug-induced hepatotoxicity. Exp. Opin. Drug Metab. Toxicol. 2013;9:589–616. doi: 10.1517/17425255.2013.773973. - DOI - PubMed
LinkOut - more resources
Full Text Sources

