Global Molecular Epidemiology of Respiratory Syncytial Virus from the 2017-2018 INFORM-RSV Study
- PMID: 33087438
- PMCID: PMC7771447
- DOI: 10.1128/JCM.01828-20
Global Molecular Epidemiology of Respiratory Syncytial Virus from the 2017-2018 INFORM-RSV Study
Abstract
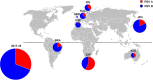
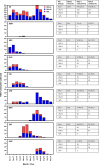
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infection among infants and young children, resulting in annual epidemics worldwide. INFORM-RSV is a multiyear clinical study designed to describe the global molecular epidemiology of RSV in children under 5 years of age by monitoring temporal and geographical evolution of current circulating RSV strains, F protein antigenic sites, and their relationships with clinical features of RSV disease. During the pilot season (2017-2018), 410 RSV G-F gene sequences were obtained from 476 RSV-positive nasal samples collected from 8 countries (United Kingdom, Spain, The Netherlands, Finland, Japan, Brazil, South Africa, and Australia). RSV B (all BA9 genotype) predominated over RSV A (all ON1 genotype) globally (69.0% versus 31.0%) and in all countries except South Africa. Geographic clustering patterns highlighted wide transmission and continued evolution with viral spread. Most RSV strains were from infants of <1 year of age (81.2%), males (56.3%), and patients hospitalized for >24 h (70.5%), with no differences in subtype distribution. Compared to 2013 reference sequences, variations at F protein antigenic sites were observed for both RSV A and B strains, with high-frequency polymorphisms at antigenic site Ø (I206M/Q209R) and site V (L172Q/S173L/K191R) in RSV B strains. The INFORM-RSV 2017-2018 pilot season establishes an important molecular baseline of RSV strain distribution and sequence variability with which to track the emergence of new strains and provide an early warning system of neutralization escape variants that may impact transmission or the effectiveness of vaccines and MAbs under development.
Keywords: evolution; genetic variation; molecular epidemiology; resistance; respiratory syncytial virus; surveillance.
Copyright © 2020 Tabor et al.
Figures
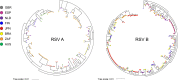

Similar articles
-
Characterization of human respiratory syncytial virus (RSV) isolated from HIV-exposed-uninfected and HIV-unexposed infants in South Africa during 2015-2017.Influenza Other Respir Viruses. 2020 Jul;14(4):403-411. doi: 10.1111/irv.12727. Epub 2020 Mar 3. Influenza Other Respir Viruses. 2020. PMID: 32126161 Free PMC article.
-
Molecular Evolution of Attachment Glycoprotein (G) and Fusion Protein (F) Genes of Respiratory Syncytial Virus ON1 and BA9 Strains in Xiamen, China.Microbiol Spectr. 2022 Apr 27;10(2):e0208321. doi: 10.1128/spectrum.02083-21. Epub 2022 Mar 21. Microbiol Spectr. 2022. PMID: 35311585 Free PMC article.
-
Epidemiology and Molecular Characterization of Human Respiratory Syncytial Virus in Senegal after Four Consecutive Years of Surveillance, 2012-2015.PLoS One. 2016 Jun 17;11(6):e0157163. doi: 10.1371/journal.pone.0157163. eCollection 2016. PLoS One. 2016. PMID: 27315120 Free PMC article.
-
Molecular epidemiology of respiratory syncytial virus.Rev Med Virol. 2001 Mar-Apr;11(2):103-16. doi: 10.1002/rmv.305. Rev Med Virol. 2001. PMID: 11262529 Review.
-
Prevalence of human respiratory syncytial virus circulating in Iran.J Infect Public Health. 2016 Mar-Apr;9(2):125-35. doi: 10.1016/j.jiph.2015.05.005. Epub 2015 Jul 2. J Infect Public Health. 2016. PMID: 26143136 Review.
Cited by
-
Detailed Molecular Interactions between Respiratory Syncytial Virus Fusion Protein and the TLR4/MD-2 Complex In Silico.Viruses. 2022 Oct 28;14(11):2382. doi: 10.3390/v14112382. Viruses. 2022. PMID: 36366480 Free PMC article.
-
Respiratory Syncytial Virus in Adult Patients at a Tertiary Care Hospital in Germany: Clinical Features and Molecular Epidemiology of the Fusion Protein in the Severe Respiratory Season of 2022/2023.Viruses. 2024 Jun 12;16(6):943. doi: 10.3390/v16060943. Viruses. 2024. PMID: 38932235 Free PMC article.
-
Respiratory syncytial virus-associated hospitalizations among children: an Italian retrospective observational study.Ital J Pediatr. 2024 Mar 7;50(1):45. doi: 10.1186/s13052-024-01617-w. Ital J Pediatr. 2024. PMID: 38454523 Free PMC article.
-
In-Depth Analysis of the Re-Emergence of Respiratory Syncytial Virus at a Tertiary Care Hospital in Germany in the Summer of 2021 after the Alleviation of Non-Pharmaceutical Interventions Due to the SARS-CoV-2 Pandemic.Viruses. 2023 Mar 29;15(4):877. doi: 10.3390/v15040877. Viruses. 2023. PMID: 37112857 Free PMC article.
-
Respiratory syncytial virus-associated hospitalization in children aged <2 years in Spain from 2018 to 2021.Hum Vaccin Immunother. 2023 Aug 1;19(2):2231818. doi: 10.1080/21645515.2023.2231818. Epub 2023 Jul 12. Hum Vaccin Immunother. 2023. PMID: 37435824 Free PMC article.
References
-
- Shi T, McAllister DA, O'Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, Alassani I, Ali A, Antonio M, Awasthi S, Awori JO, Azziz-Baumgartner E, Baggett HC, Baillie VL, Balmaseda A, Barahona A, Basnet S, Bassat Q, Basualdo W, Bigogo G, Bont LJ, Breiman RF, Brooks WA, Broor S, Bruce N, Bruden D, Buchy P, Campbell S, Carosone-Link P, Chadha M, Chipeta J, Chou M, Clara W, Cohen C, de Cuellar E, Dang D-A, Dash-Yandag B, Deloria-Knoll M, Dherani M, Eap T, Ebruke BE, Echavarria M, de Freitas Lázaro Emediato CC, Fasce RA, Feikin DR, Feng L, et al. . 2017. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 390:946–958. doi:10.1016/S0140-6736(17)30938-8. - DOI - PMC - PubMed
-
- Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, Rodriguez-Tenreiro C, Sly P, Ramilo O, Mejias A, Baraldi E, Papadopoulos NG, Nair H, Nunes MC, Kragten-Tabatabaie L, Heikkinen T, Greenough A, Stein RT, Manzoni P, Bont LJ, Martinon-Torres F. 2018. Respiratory syncytial virus seasonality: a global overview. J Infect Dis 217:1356–1364. doi:10.1093/infdis/jiy056. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

