Autophagosome formation in relation to the endoplasmic reticulum
- PMID: 33087127
- PMCID: PMC7579975
- DOI: 10.1186/s12929-020-00691-6
Autophagosome formation in relation to the endoplasmic reticulum
Abstract
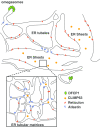
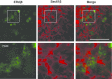
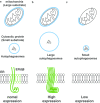
Autophagy is a process in which a myriad membrane structures called autophagosomes are formed de novo in a single cell, which deliver the engulfed substrates into lysosomes for degradation. The size of the autophagosomes is relatively uniform in non-selective autophagy and variable in selective autophagy. It has been recently established that autophagosome formation occurs near the endoplasmic reticulum (ER). In this review, we have discussed recent advances in the relationship between autophagosome formation and endoplasmic reticulum. Autophagosome formation occurs near the ER subdomain enriched with phospholipid synthesizing enzymes like phosphatidylinositol synthase (PIS)/CDP-diacylglycerol-inositol 3-phosphatidyltransferase (CDIPT) and choline/ethanolamine phosphotransferase 1 (CEPT1). Autophagy-related protein 2 (Atg2), which is involved in autophagosome formation has a lipid transfer capacity and is proposed to directly transfer the lipid molecules from the ER to form autophagosomes. Vacuole membrane protein 1 (VMP1) and transmembrane protein 41b (TMEM41b) are ER membrane proteins that are associated with the formation of the subdomain. Recently, we have reported that an uncharacterized ER membrane protein possessing the DNAJ domain, called ERdj8/DNAJC16, is associated with the regulation of the size of autophagosomes. The localization of ERdj8/DNAJC16 partially overlaps with the PIS-enriched ER subdomain, thereby implying its association with autophagosome size determination.
Keywords: ATG9; Atg2; Autophagosome; Autophagy; CDIPT; COPII; ERdj8/DNAJC16; Endoplasmic reticulum; PIS; TMEM41b; VMP1.
Conflict of interest statement
There are no competing interests to declare.
Figures
Similar articles
-
ERdj8 governs the size of autophagosomes during the formation process.J Cell Biol. 2020 Aug 3;219(8):e201903127. doi: 10.1083/jcb.201903127. J Cell Biol. 2020. PMID: 32492081 Free PMC article.
-
Autophagosome formation is initiated at phosphatidylinositol synthase-enriched ER subdomains.EMBO J. 2017 Jun 14;36(12):1719-1735. doi: 10.15252/embj.201695189. Epub 2017 May 11. EMBO J. 2017. PMID: 28495679 Free PMC article.
-
Lipid Transport from Endoplasmic Reticulum to Autophagic Membranes.Cold Spring Harb Perspect Biol. 2022 Nov 1;14(11):a041254. doi: 10.1101/cshperspect.a041254. Cold Spring Harb Perspect Biol. 2022. PMID: 35940912 Free PMC article. Review.
-
Autophagosome Biogenesis and the Endoplasmic Reticulum: A Plant Perspective.Trends Plant Sci. 2018 Aug;23(8):677-692. doi: 10.1016/j.tplants.2018.05.002. Epub 2018 Jun 18. Trends Plant Sci. 2018. PMID: 29929776 Review.
-
ATG9 regulates autophagosome progression from the endoplasmic reticulum in Arabidopsis.Proc Natl Acad Sci U S A. 2017 Jan 17;114(3):E426-E435. doi: 10.1073/pnas.1616299114. Epub 2017 Jan 4. Proc Natl Acad Sci U S A. 2017. PMID: 28053229 Free PMC article.
Cited by
-
Dysregulation of Autophagy Occurs During Congenital Cataract Development in βA3ΔG91 Mice.Invest Ophthalmol Vis Sci. 2024 Apr 1;65(4):4. doi: 10.1167/iovs.65.4.4. Invest Ophthalmol Vis Sci. 2024. PMID: 38558092 Free PMC article.
-
Effects of Visceralising Leishmania on the Spleen, Liver, and Bone Marrow: A Pathophysiological Perspective.Microorganisms. 2021 Apr 5;9(4):759. doi: 10.3390/microorganisms9040759. Microorganisms. 2021. PMID: 33916346 Free PMC article. Review.
-
The Role of Retinal Pigment Epithelial Cells in Age-Related Macular Degeneration: Phagocytosis and Autophagy.Biomolecules. 2023 May 29;13(6):901. doi: 10.3390/biom13060901. Biomolecules. 2023. PMID: 37371481 Free PMC article. Review.
-
Research progress on the relationship between autophagy and chronic complications of diabetes.Front Physiol. 2022 Aug 8;13:956344. doi: 10.3389/fphys.2022.956344. eCollection 2022. Front Physiol. 2022. PMID: 36003645 Free PMC article. Review.
-
Abnormal Vacuole Membrane Protein-1 Expression in Parkinson's Disease Patients.Front Neurosci. 2022 Apr 6;16:760932. doi: 10.3389/fnins.2022.760932. eCollection 2022. Front Neurosci. 2022. PMID: 35464320 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

