IBL-202 is synergistic with venetoclax in CLL under in vitro conditions that mimic the tumor microenvironment
- PMID: 33085757
- PMCID: PMC7594376
- DOI: 10.1182/bloodadvances.2019001369
IBL-202 is synergistic with venetoclax in CLL under in vitro conditions that mimic the tumor microenvironment
Abstract
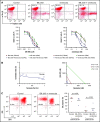
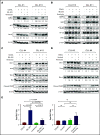
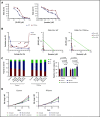
The B-cell receptor signaling pathway and dysregulation of the Bcl-2 family of proteins play crucial roles in the pathogenesis of chronic lymphocytic leukemia (CLL). Despite significant advances in the treatment of the disease, relapse and drug resistance are not uncommon. In the current study, we investigated the dual PI3/PIM kinase inhibitor IBL-202 in combination with venetoclax as a treatment option for CLL using both primary CLL cells and TP53-deficient OSU-CLL cells generated using the CRISPR-Cas9 system. IBL-202 and venetoclax were highly synergistic against primary CLL cells cocultured with CD40L fibroblasts (combination index [CI], 0.4, at a fractional effect of 0.9) and TP53-knockout (KO) OSU-CLL cells (CI, 0.5, at a fractional effect of 0.9). Synergy between the drugs was consistent, with a significant (P < .05) reduction in the 50% inhibitory concentration for both drugs. IBL-202 and venetoclax in combination induced cell-cycle arrest and slowed the proliferation of both wild-type and TP53-KO cell lines. The drug combination inhibited AKT phosphorylation, reduced expression of Bcl-xL and NF-κB, and increased the Noxa/Mcl-1 ratio. Downregulation of CXCR4 was consistent with inhibition of the SDF-1α-induced migratory capacity of CLL cells. Synergy between IBL-202 and venetoclax against primary CLL cells cultured under conditions that mimic the tumor microenvironment suggests this drug combination may be effective against CLL cells within the lymph nodes and bone marrow. Furthermore, the efficacy of the combination against the TP53-KO OSU-CLL cell line suggests the combination may be a highly effective treatment strategy for high-risk CLL.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
Similar articles
-
MEK1/2 inhibition by binimetinib is effective as a single agent and potentiates the actions of Venetoclax and ABT-737 under conditions that mimic the chronic lymphocytic leukaemia (CLL) tumour microenvironment.Br J Haematol. 2018 Aug;182(3):360-372. doi: 10.1111/bjh.15282. Epub 2018 May 16. Br J Haematol. 2018. PMID: 29767411
-
The dual inhibitor of the phosphoinositol-3 and PIM kinases, IBL-202, is effective against chronic lymphocytic leukaemia cells under conditions that mimic the hypoxic tumour microenvironment.Br J Haematol. 2018 Sep;182(5):654-669. doi: 10.1111/bjh.15447. Epub 2018 Jul 5. Br J Haematol. 2018. PMID: 29978459
-
The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53-independent mechanism.Blood. 2016 Jun 23;127(25):3215-24. doi: 10.1182/blood-2016-01-688796. Epub 2016 Apr 11. Blood. 2016. PMID: 27069256 Free PMC article.
-
Venetoclax for the treatment of chronic lymphocytic leukemia.Expert Opin Investig Drugs. 2017 Nov;26(11):1307-1316. doi: 10.1080/13543784.2017.1386173. Epub 2017 Oct 9. Expert Opin Investig Drugs. 2017. PMID: 28972395 Review.
-
Potential of BCL2 as a target for chronic lymphocytic leukemia treatment.Expert Rev Hematol. 2018 May;11(5):391-402. doi: 10.1080/17474086.2018.1456332. Epub 2018 Mar 29. Expert Rev Hematol. 2018. PMID: 29561706 Review.
Cited by
-
PIM Kinases in Multiple Myeloma.Cancers (Basel). 2021 Aug 26;13(17):4304. doi: 10.3390/cancers13174304. Cancers (Basel). 2021. PMID: 34503111 Free PMC article. Review.
-
In Vitro and In Vivo Models of CLL-T Cell Interactions: Implications for Drug Testing.Cancers (Basel). 2022 Jun 23;14(13):3087. doi: 10.3390/cancers14133087. Cancers (Basel). 2022. PMID: 35804862 Free PMC article. Review.
References
-
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352(8):804-815. - PubMed
-
- Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96(8):2655-2663. - PubMed
-
- Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615-675. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

