Hypoxia-autophagy axis induces VEGFA by peritoneal mesothelial cells to promote gastric cancer peritoneal metastasis through an integrin α5-fibronectin pathway
- PMID: 33081836
- PMCID: PMC7576728
- DOI: 10.1186/s13046-020-01703-x
Hypoxia-autophagy axis induces VEGFA by peritoneal mesothelial cells to promote gastric cancer peritoneal metastasis through an integrin α5-fibronectin pathway
Abstract
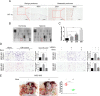
Background: Peritoneal metastasis (PM) is an important pathological process in the progression of gastric cancer (GC). The metastatic potential of tumor and stromal cells is governed by hypoxia, which is a key molecular feature of the tumor microenvironment. Mesothelial cells also participate in this complex and dynamic process. However, the molecular mechanisms underlying the hypoxia-driven mesothelial-tumor interactions that promote peritoneal metastasis of GC remain unclear.
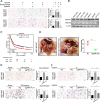
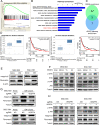
Methods: We determined the hypoxic microenvironment in PM of nude mice by immunohistochemical analysis and screened VEGFA by human growth factor array kit. The crosstalk mediated by VEGFA between peritoneal mesothelial cells (PMCs) and GC cells was determined in GC cells incubated with conditioned medium prepared from hypoxia-treated PMCs. The association between VEGFR1 and integrin α5 and fibronectin in GC cells was enriched using Gene Set Enrichment Analysis and KEGG pathway enrichment analysis. In vitro and xenograft mouse models were used to evaluate the impact of VEGFA/VEGFR1 on gastric cancer peritoneal metastasis. Confocal microscopy and immunoprecipitation were performed to determine the effect of hypoxia-induced autophagy.
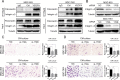
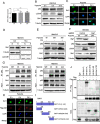
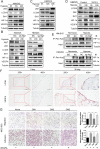
Results: Here we report that in the PMCs of the hypoxic microenvironment, SIRT1 is degraded via the autophagic lysosomal pathway, leading to increased acetylation of HIF-1α and secretion of VEGFA. Under hypoxic conditions, VEGFA derived from PMCs acts on VEGFR1 of GC cells, resulting in p-ERK/p-JNK pathway activation, increased integrin α5 and fibronectin expression, and promotion of PM.
Conclusions: Our findings have elucidated the mechanisms by which PMCs promote PM in GC in hypoxic environments. This study also provides a theoretical basis for considering autophagic pathways or VEGFA as potential therapeutic targets to treat PM in GC.
Keywords: Adhesion; Autophagy; Hypoxia; Migration; VEGFA.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures
Similar articles
-
Exosomal miR-21-5p derived from gastric cancer promotes peritoneal metastasis via mesothelial-to-mesenchymal transition.Cell Death Dis. 2018 Aug 28;9(9):854. doi: 10.1038/s41419-018-0928-8. Cell Death Dis. 2018. PMID: 30154401 Free PMC article.
-
Exosomal miR-106a derived from gastric cancer promotes peritoneal metastasis via direct regulation of Smad7.Cell Cycle. 2020 May;19(10):1200-1221. doi: 10.1080/15384101.2020.1749467. Epub 2020 Apr 8. Cell Cycle. 2020. PMID: 32267797 Free PMC article.
-
Collagen Remodeling in the Hypoxic Tumor-Mesothelial Niche Promotes Ovarian Cancer Metastasis.Cancer Res. 2019 May 1;79(9):2271-2284. doi: 10.1158/0008-5472.CAN-18-2616. Epub 2019 Mar 12. Cancer Res. 2019. PMID: 30862717 Free PMC article.
-
Alteration and dysfunction of ion channels/transporters in a hypoxic microenvironment results in the development and progression of gastric cancer.Cell Oncol (Dordr). 2021 Aug;44(4):739-749. doi: 10.1007/s13402-021-00604-1. Epub 2021 Apr 15. Cell Oncol (Dordr). 2021. PMID: 33856653 Free PMC article. Review.
-
Understanding the "lethal" drivers of tumor-stroma co-evolution: emerging role(s) for hypoxia, oxidative stress and autophagy/mitophagy in the tumor micro-environment.Cancer Biol Ther. 2010 Sep 15;10(6):537-42. doi: 10.4161/cbt.10.6.13370. Epub 2010 Sep 19. Cancer Biol Ther. 2010. PMID: 20861671 Free PMC article. Review.
Cited by
-
Ovarian hormones-autophagy-immunity axis in menstruation and endometriosis.Theranostics. 2021 Jan 19;11(7):3512-3526. doi: 10.7150/thno.55241. eCollection 2021. Theranostics. 2021. PMID: 33537101 Free PMC article. Review.
-
ACLY promotes gastric tumorigenesis and accelerates peritoneal metastasis of gastric cancer regulated by HIF-1A.Cell Cycle. 2023 Oct;22(20):2288-2301. doi: 10.1080/15384101.2023.2286805. Epub 2023 Dec 15. Cell Cycle. 2023. PMID: 38009671 Free PMC article.
-
The modulation of ion channels in cancer chemo-resistance.Front Oncol. 2022 Aug 10;12:945896. doi: 10.3389/fonc.2022.945896. eCollection 2022. Front Oncol. 2022. PMID: 36033489 Free PMC article. Review.
-
ITGA5 is associated with prognosis marker and immunosuppression in head and neck squamous cell carcinoma.Diagn Pathol. 2024 Oct 7;19(1):134. doi: 10.1186/s13000-024-01559-1. Diagn Pathol. 2024. PMID: 39375732 Free PMC article.
-
Development of the Peritoneal Metastasis: A Review of Back-Grounds, Mechanisms, Treatments and Prospects.J Clin Med. 2022 Dec 23;12(1):103. doi: 10.3390/jcm12010103. J Clin Med. 2022. PMID: 36614904 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- 2016YFC1302400/the National Key R&D Program of China
- IRT_17R107&IRT13101/The Ministry of education innovation team development plan
- 81130042, 31171323, 81770001/Key project of the National Natural Science Foundation
- NO.8197103748/National Natural Science Foundation of China
- 81803092/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

