Antibody-Drug Conjugates for Cancer Therapy
- PMID: 33081383
- PMCID: PMC7587605
- DOI: 10.3390/molecules25204764
Antibody-Drug Conjugates for Cancer Therapy
Abstract
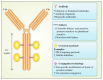
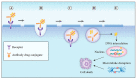
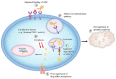
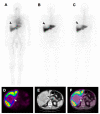
Antibody-drug conjugates (ADCs) are novel drugs that exploit the specificity of a monoclonal antibody (mAb) to reach target antigens expressed on cancer cells for the delivery of a potent cytotoxic payload. ADCs provide a unique opportunity to deliver drugs to tumor cells while minimizing toxicity to normal tissue, achieving wider therapeutic windows and enhanced pharmacokinetic/pharmacodynamic properties. To date, nine ADCs have been approved by the FDA and more than 80 ADCs are under clinical development worldwide. In this paper, we provide an overview of the biology and chemistry of each component of ADC design. We briefly discuss the clinical experience with approved ADCs and the various pathways involved in ADC resistance. We conclude with perspectives about the future development of the next generations of ADCs, including the role of molecular imaging in drug development.
Keywords: ADC; antibody–drug conjugate; cytotoxic payload; linkers, cancer; molecular imaging.; monoclonal antibody.
Conflict of interest statement
Andrew Scott has consulting roles and research funding from EMD Serono, Abbvie, Astra Zeneca, Telix, Medimmune, Adalta, ITM, and Imagion Bio; he is a consultant to Life Science Pharmaceuticals.
Figures
Similar articles
-
Antibody-Drug Conjugates: The New Frontier of Chemotherapy.Int J Mol Sci. 2020 Jul 31;21(15):5510. doi: 10.3390/ijms21155510. Int J Mol Sci. 2020. PMID: 32752132 Free PMC article. Review.
-
Recent Chemical Approaches for Site-Specific Conjugation of Native Antibodies: Technologies toward Next-Generation Antibody-Drug Conjugates.Chembiochem. 2019 Nov 4;20(21):2729-2737. doi: 10.1002/cbic.201900178. Epub 2019 Jul 17. Chembiochem. 2019. PMID: 30973187 Review.
-
Antibody-drug conjugates: Principles and opportunities.Life Sci. 2024 Jun 15;347:122676. doi: 10.1016/j.lfs.2024.122676. Epub 2024 Apr 28. Life Sci. 2024. PMID: 38688384 Review.
-
Picking the optimal target for antibody-drug conjugates.Am Soc Clin Oncol Educ Book. 2013. doi: 10.14694/EdBook_AM.2013.33.e103. Am Soc Clin Oncol Educ Book. 2013. PMID: 23714470 Review.
-
Antibody-drug conjugates: Promising and efficient tools for targeted cancer therapy.J Cell Physiol. 2018 Sep;233(9):6441-6457. doi: 10.1002/jcp.26435. Epub 2018 Mar 25. J Cell Physiol. 2018. PMID: 29319167 Review.
Cited by
-
Current status of immunotherapy for non-small cell lung cancer.Front Pharmacol. 2022 Oct 13;13:989461. doi: 10.3389/fphar.2022.989461. eCollection 2022. Front Pharmacol. 2022. PMID: 36313314 Free PMC article. Review.
-
A promising target for breast cancer: B7-H3.BMC Cancer. 2024 Feb 7;24(1):182. doi: 10.1186/s12885-024-11933-3. BMC Cancer. 2024. PMID: 38326735 Free PMC article. Review.
-
The present and future of immunocytokines for cancer treatment.Cell Mol Life Sci. 2022 Sep 6;79(10):509. doi: 10.1007/s00018-022-04514-9. Cell Mol Life Sci. 2022. PMID: 36066630 Free PMC article. Review.
-
Antibody-drug conjugates in urinary tumors: clinical application, challenge, and perspectives.Front Oncol. 2023 Dec 20;13:1259784. doi: 10.3389/fonc.2023.1259784. eCollection 2023. Front Oncol. 2023. PMID: 38173833 Free PMC article. Review.
-
Synthesis and biological evaluation of novel quaternary ammonium antibody drug conjugates based on camptothecin derivatives.PLoS One. 2023 Dec 19;18(12):e0292871. doi: 10.1371/journal.pone.0292871. eCollection 2023. PLoS One. 2023. PMID: 38113206 Free PMC article.
References
-
- Factsheet W. Cancer. [(accessed on 29 November 2019)]; Available online: https://www.who.int/news-room/fact-sheets/detail/cancer.
-
- Davis C., Naci H., Gurpinar E., Poplavska E., Pinto A., Aggarwal A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: Retrospective cohort study of drug approvals 2009–13. BMJ. 2017;359:j4530. doi: 10.1136/bmj.j4530. - DOI - PMC - PubMed
-
- Sharma V. CPhI Annual Report 2018: ADCs Growth Driven by Lack of In-House Facilities, Oncology and Integrated CDMOs. [(accessed on 7 July 2020)]; Available online: https://www.pharmoutsourcing.com/Featured-Articles/354437-CPhI-Annual-Re...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

