Expansion of Intrinsically Disordered Proteins Increases the Range of Stability of Liquid-Liquid Phase Separation
- PMID: 33076213
- PMCID: PMC7587599
- DOI: 10.3390/molecules25204705
Expansion of Intrinsically Disordered Proteins Increases the Range of Stability of Liquid-Liquid Phase Separation
Abstract
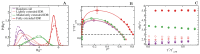
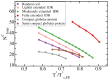
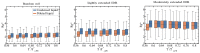
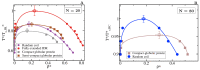
Proteins containing intrinsically disordered regions (IDRs) are ubiquitous within biomolecular condensates, which are liquid-like compartments within cells formed through liquid-liquid phase separation (LLPS). The sequence of amino acids of a protein encodes its phase behaviour, not only by establishing the patterning and chemical nature (e.g., hydrophobic, polar, charged) of the various binding sites that facilitate multivalent interactions, but also by dictating the protein conformational dynamics. Besides behaving as random coils, IDRs can exhibit a wide-range of structural behaviours, including conformational switching, where they transition between alternate conformational ensembles. Using Molecular Dynamics simulations of a minimal coarse-grained model for IDRs, we show that the role of protein conformation has a non-trivial effect in the liquid-liquid phase behaviour of IDRs. When an IDR transitions to a conformational ensemble enriched in disordered extended states, LLPS is enhanced. In contrast, IDRs that switch to ensembles that preferentially sample more compact and structured states show inhibited LLPS. This occurs because extended and disordered protein conformations facilitate LLPS-stabilising multivalent protein-protein interactions by reducing steric hindrance; thereby, such conformations maximize the molecular connectivity of the condensed liquid network. Extended protein configurations promote phase separation regardless of whether LLPS is driven by homotypic and/or heterotypic protein-protein interactions. This study sheds light on the link between the dynamic conformational plasticity of IDRs and their liquid-liquid phase behaviour.
Keywords: biological phase transitions; computer simulations; proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Classification of proteins inducing liquid-liquid phase separation: sequential, structural and functional characterization.J Biochem. 2023 Mar 31;173(4):255-264. doi: 10.1093/jb/mvac106. J Biochem. 2023. PMID: 36575582 Free PMC article.
-
Liquid-Liquid Phase Separation by Intrinsically Disordered Protein Regions of Viruses: Roles in Viral Life Cycle and Control of Virus-Host Interactions.Int J Mol Sci. 2020 Nov 28;21(23):9045. doi: 10.3390/ijms21239045. Int J Mol Sci. 2020. PMID: 33260713 Free PMC article. Review.
-
Targeted modulation of protein liquid-liquid phase separation by evolution of amino-acid sequence.PLoS Comput Biol. 2021 Aug 24;17(8):e1009328. doi: 10.1371/journal.pcbi.1009328. eCollection 2021 Aug. PLoS Comput Biol. 2021. PMID: 34428231 Free PMC article.
-
A call to order: Examining structured domains in biomolecular condensates.J Magn Reson. 2023 Jan;346:107318. doi: 10.1016/j.jmr.2022.107318. J Magn Reson. 2023. PMID: 36657879 Free PMC article.
-
Towards Decoding the Sequence-Based Grammar Governing the Functions of Intrinsically Disordered Protein Regions.J Mol Biol. 2021 Jun 11;433(12):166724. doi: 10.1016/j.jmb.2020.11.023. Epub 2020 Nov 26. J Mol Biol. 2021. PMID: 33248138 Review.
Cited by
-
The effect of polymer length in liquid-liquid phase separation.Cell Rep Phys Sci. 2023 May 17;4(5):101415. doi: 10.1016/j.xcrp.2023.101415. Epub 2023 May 8. Cell Rep Phys Sci. 2023. PMID: 37325682 Free PMC article.
-
Protein structural transitions critically transform the network connectivity and viscoelasticity of RNA-binding protein condensates but RNA can prevent it.Nat Commun. 2022 Sep 29;13(1):5717. doi: 10.1038/s41467-022-32874-0. Nat Commun. 2022. PMID: 36175408 Free PMC article.
-
Thermodynamics and kinetics of phase separation of protein-RNA mixtures by a minimal model.Biophys J. 2021 Apr 6;120(7):1219-1230. doi: 10.1016/j.bpj.2021.01.031. Epub 2021 Feb 9. Biophys J. 2021. PMID: 33571491 Free PMC article.
-
Valency and Binding Affinity Variations Can Regulate the Multilayered Organization of Protein Condensates with Many Components.Biomolecules. 2021 Feb 14;11(2):278. doi: 10.3390/biom11020278. Biomolecules. 2021. PMID: 33672806 Free PMC article.
-
LLPS vs. LLCPS: analogies and differences.Soft Matter. 2023 Mar 8;19(10):1873-1881. doi: 10.1039/d2sm01455f. Soft Matter. 2023. PMID: 36806460 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

