Advanced glycation end-products disrupt brain microvascular endothelial cell barrier: The role of mitochondria and oxidative stress
- PMID: 33075405
- PMCID: PMC8782206
- DOI: 10.1016/j.mvr.2020.104098
Advanced glycation end-products disrupt brain microvascular endothelial cell barrier: The role of mitochondria and oxidative stress
Abstract
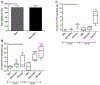
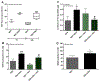
During diabetes mellitus, advanced glycation end-products (AGEs) are major contributors to the development of alterations in cerebral capillaries, leading to the disruption of the blood-brain barrier (BBB). Consequently, this is often associated with an amplified oxidative stress response in microvascular endothelial cells. As a model to mimic brain microvasculature, the bEnd.3 endothelial cell line was used to investigate cell barrier function. Cells were exposed to native bovine serum albumin (BSA) or modified BSA (BSA-AGEs). In the presence or absence of the antioxidant compound, N-acetyl-cysteine, cell permeability was assessed by FITC-dextran exclusion, intracellular free radical formation was monitored with H2DCF-DA probe, and mitochondrial respiratory and redox parameters were analyzed. We report that, in the absence of alterations in cell viability, BSA-AGEs contribute to an increase in endothelial cell barrier permeability and a marked and prolonged oxidative stress response. Decreased mitochondrial oxygen consumption was associated with these alterations and may contribute to reactive oxygen species production. These results suggest the need for further research to explore therapeutic interventions to restore mitochondrial functionality in microvascular endothelial cells to improve brain homeostasis in pathological complications associated with glycation.
Keywords: Advanced glycation end-products; Diabetes; Endothelial dysfunction; Mitochondria; Oxidative stress.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interests
The authors declare that there are no conflicts of interests
Figures

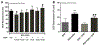


Similar articles
-
Advanced glycation end-products disrupt human endothelial cells redox homeostasis: new insights into reactive oxygen species production.Free Radic Res. 2019 Feb;53(2):150-169. doi: 10.1080/10715762.2018.1529866. Epub 2019 Mar 1. Free Radic Res. 2019. PMID: 30821539
-
Mdia1 is Crucial for Advanced Glycation End Product-Induced Endothelial Hyperpermeability.Cell Physiol Biochem. 2018;45(4):1717-1730. doi: 10.1159/000487780. Epub 2018 Feb 23. Cell Physiol Biochem. 2018. PMID: 29490301
-
Matrine-Type Alkaloids Inhibit Advanced Glycation End Products Induced Reactive Oxygen Species-Mediated Apoptosis of Aortic Endothelial Cells In Vivo and In Vitro by Targeting MKK3 and p38MAPK Signaling.J Am Heart Assoc. 2017 Dec 2;6(12):e007441. doi: 10.1161/JAHA.117.007441. J Am Heart Assoc. 2017. PMID: 29197828 Free PMC article.
-
Coronary microvascular injury in myocardial infarction: perception and knowledge for mitochondrial quality control.Theranostics. 2021 May 3;11(14):6766-6785. doi: 10.7150/thno.60143. eCollection 2021. Theranostics. 2021. PMID: 34093852 Free PMC article. Review.
-
Mitochondrial oxidative stress in brain microvascular endothelial cells: Triggering blood-brain barrier disruption.Mitochondrion. 2023 Mar;69:71-82. doi: 10.1016/j.mito.2023.01.007. Epub 2023 Jan 26. Mitochondrion. 2023. PMID: 36709855 Review.
Cited by
-
Impact of Enhanced Phagocytosis of Glycated Erythrocytes on Human Endothelial Cell Functions.Cells. 2022 Jul 14;11(14):2200. doi: 10.3390/cells11142200. Cells. 2022. PMID: 35883644 Free PMC article.
-
A Role for Advanced Glycation End Products in Molecular Ageing.Int J Mol Sci. 2023 Jun 8;24(12):9881. doi: 10.3390/ijms24129881. Int J Mol Sci. 2023. PMID: 37373042 Free PMC article. Review.
-
Glucose Uptake by Skeletal Muscle within the Contexts of Type 2 Diabetes and Exercise: An Integrated Approach.Nutrients. 2022 Feb 3;14(3):647. doi: 10.3390/nu14030647. Nutrients. 2022. PMID: 35277006 Free PMC article. Review.
-
Neuroprotective Effects of Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors (Gliflozins) on Diabetes-Induced Neurodegeneration and Neurotoxicity: A Graphical Review.Int J Prev Med. 2024 Aug 6;15:28. doi: 10.4103/ijpvm.ijpvm_5_23. eCollection 2024. Int J Prev Med. 2024. PMID: 39239308 Free PMC article. Review.
-
Microglia at the blood brain barrier in health and disease.Front Cell Neurosci. 2024 Mar 13;18:1360195. doi: 10.3389/fncel.2024.1360195. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38550920 Free PMC article. Review.
References
-
- Aouacheri O, et al., 2015. The investigation of the oxidative stress-related parameters in type 2 diabetes mellitus. Can J Diabetes. 39, 44–9. - PubMed
-
- Banks WA, 2020. The Blood-Brain Barrier Interface in Diabetes Mellitus: Dysfunctions, Mechanisms and Approaches to Treatment. Curr Pharm Des. 26, 1438–1447. - PubMed
-
- Baraka-Vidot J, et al., 2015. Glycation alters ligand binding, enzymatic, and pharmacological properties of human albumin. Biochemistry. 54, 3051–62. - PubMed
-
- Baret P, et al., 2018. Glycated human albumin alters mitochondrial respiration in preadipocyte 3T3-L1 cells. Biofactors. 43, 577–592. - PubMed
-
- Brosnan C, Claudio L, Brain microvasculature in multiple sclerosis. In: Pardridge WM, (Ed.), Introduction to the blood-brain barrier: methodology, biology and pathology. Cambridge University Press, cop. 1998., Cambridge; New York: 1998.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

