Cellular Signaling Pathways in Medium and Large Vessel Vasculitis
- PMID: 33072134
- PMCID: PMC7544845
- DOI: 10.3389/fimmu.2020.587089
Cellular Signaling Pathways in Medium and Large Vessel Vasculitis
Abstract
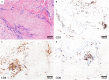
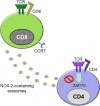
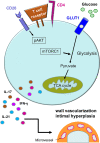
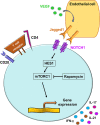
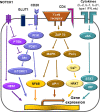
Autoimmune and autoinflammatory diseases of the medium and large arteries, including the aorta, cause life-threatening complications due to vessel wall destruction but also by wall remodeling, such as the formation of wall-penetrating microvessels and lumen-stenosing neointima. The two most frequent large vessel vasculitides, giant cell arteritis (GCA) and Takayasu arteritis (TAK), are HLA-associated diseases, strongly suggestive for a critical role of T cells and antigen recognition in disease pathogenesis. Recent studies have revealed a growing spectrum of effector functions through which T cells participate in the immunopathology of GCA and TAK; causing the disease-specific patterning of pathology and clinical outcome. Core pathogenic features of disease-relevant T cells rely on the interaction with endothelial cells, dendritic cells and macrophages and lead to vessel wall invasion, formation of tissue-damaging granulomatous infiltrates and induction of the name-giving multinucleated giant cells. Besides antigen, pathogenic T cells encounter danger signals in their immediate microenvironment that they translate into disease-relevant effector functions. Decisive signaling pathways, such as the AKT pathway, the NOTCH pathway, and the JAK/STAT pathway modify antigen-induced T cell activation and emerge as promising therapeutic targets to halt disease progression and, eventually, reset the immune system to reestablish the immune privilege of the arterial wall.
Keywords: NOTCH; T cells; Takayasu arteritis; co-stimulation; giant cell arteritis; immune checkpoint; large vessel vasculitis; macrophages.
Copyright © 2020 Watanabe, Berry, Liang, Goronzy and Weyand.
Figures

Similar articles
-
[Pathogenesis of large vessel vasculitides].Z Rheumatol. 2020 Aug;79(6):505-515. doi: 10.1007/s00393-020-00809-z. Z Rheumatol. 2020. PMID: 32430566 Review. German.
-
The Immunopathology of Giant Cell Arteritis Across Disease Spectra.Front Immunol. 2021 Feb 25;12:623716. doi: 10.3389/fimmu.2021.623716. eCollection 2021. Front Immunol. 2021. PMID: 33717128 Free PMC article. Review.
-
Pathogenesis of Giant Cell Arteritis and Takayasu Arteritis-Similarities and Differences.Curr Rheumatol Rep. 2020 Aug 26;22(10):68. doi: 10.1007/s11926-020-00948-x. Curr Rheumatol Rep. 2020. PMID: 32845392 Free PMC article. Review.
-
Inhibition of JAK-STAT Signaling Suppresses Pathogenic Immune Responses in Medium and Large Vessel Vasculitis.Circulation. 2018 May 1;137(18):1934-1948. doi: 10.1161/CIRCULATIONAHA.117.030423. Epub 2017 Dec 18. Circulation. 2018. PMID: 29254929 Free PMC article.
-
Innate and Adaptive Immunity in Giant Cell Arteritis.Front Immunol. 2021 Feb 25;11:621098. doi: 10.3389/fimmu.2020.621098. eCollection 2020. Front Immunol. 2021. PMID: 33717054 Free PMC article. Review.
Cited by
-
Immunology of Giant Cell Arteritis.Circ Res. 2023 Jan 20;132(2):238-250. doi: 10.1161/CIRCRESAHA.122.322128. Epub 2023 Jan 19. Circ Res. 2023. PMID: 36656970 Free PMC article. Review.
-
Highlights in clinical medicine-Giant cell arteritis, polymyalgia rheumatica and Takayasu's arteritis: pathogenic links and therapeutic implications.Clin Exp Med. 2022 Nov;22(4):509-518. doi: 10.1007/s10238-021-00770-4. Epub 2021 Nov 6. Clin Exp Med. 2022. PMID: 34741677 Review.
-
Neuro-ophthalmic complications of immune checkpoint inhibitor therapy: Current status and future directions.Front Ophthalmol (Lausanne). 2022 Nov 18;2:1044904. doi: 10.3389/fopht.2022.1044904. eCollection 2022. Front Ophthalmol (Lausanne). 2022. PMID: 38983573 Free PMC article. Review.
-
Rheumatic Immune-Related Adverse Events due to Immune Checkpoint Inhibitors-A 2023 Update.Int J Mol Sci. 2023 Mar 15;24(6):5643. doi: 10.3390/ijms24065643. Int J Mol Sci. 2023. PMID: 36982715 Free PMC article. Review.
-
Perspectives of JAK Inhibitors for Large Vessel Vasculitis.Front Immunol. 2022 Mar 30;13:881705. doi: 10.3389/fimmu.2022.881705. eCollection 2022. Front Immunol. 2022. PMID: 35432355 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

