Transport of Amino Acids Across the Blood-Brain Barrier
- PMID: 33071801
- PMCID: PMC7538855
- DOI: 10.3389/fphys.2020.00973
Transport of Amino Acids Across the Blood-Brain Barrier
Abstract
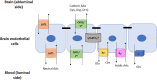
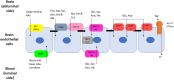
The blood-brain-barrier (BBB), present in brain capillaries, constitutes an essential barrier mechanism for normal functioning and development of the brain. The presence of tight junctions between adjacent endothelial cells restricts permeability and movement of molecules between extracellular fluid and plasma. The protein complexes that control cell-cell attachment also polarize cellular membrane, so that it can be divided into luminal (blood-facing) and abluminal (brain) sides, and each solute that enters/leaves the brain must cross both membranes. Several amino acid (AA) transport systems with different distributions on both sides of the BBB have been described. In a broad sense, there are at least five different systems of facilitative transporters and all of them are found in the luminal membrane. Some of these transporters are very specific for a small group of substrates and are located exclusively on the luminal side of the BBB. However, the two major facilitative carriers, system L and system y+, are located in both membranes, although asymmetrically. The position of these Na+-independent transporters ensures AA availability in the brain and also its bidirectional transport across the endothelial cells. On the other hand, there are several Na+-dependent transport systems that transport AAs against its concentration gradient together with the movement of Na+ ions. The majority of these active transporters are present exclusively at the abluminal membrane and are responsible for AA efflux from the brain into the endothelial cells. Since they are Na+-coupled, the sodium pump Na+/K+-ATPase is also highly expressed on this abluminal side of the BBB. Once inside the cell, the facilitative transporters located in the luminal membranes mediate export from the endothelial cell to the blood. In summary, the polarized distribution of these transport systems between the luminal and abluminal membranes, and the fact that more than one transporter may carry the same substrate, ensures supply and excretion of AAs in and out of the brain, thereby controlling its homeostasis and proper function.
Keywords: abluminal membrane; active transport; amino acid transport; blood-brain barrier; cell polarity; endothelial cells; facilitative transport; luminal membrane.
Copyright © 2020 Zaragozá.
Figures
Similar articles
-
Structure of the blood-brain barrier and its role in the transport of amino acids.J Nutr. 2006 Jan;136(1 Suppl):218S-26S. doi: 10.1093/jn/136.1.218S. J Nutr. 2006. PMID: 16365086 Review.
-
Structure of the Blood Brain Barrier and its Role in the Transporters for the Movement of Substrates across the Barriers.Curr Drug Metab. 2023;24(4):250-269. doi: 10.2174/1389200224666230608110349. Curr Drug Metab. 2023. PMID: 37291784
-
How Glutamate Is Managed by the Blood-Brain Barrier.Biology (Basel). 2016 Oct 8;5(4):37. doi: 10.3390/biology5040037. Biology (Basel). 2016. PMID: 27740595 Free PMC article. Review.
-
The complementary membranes forming the blood-brain barrier.IUBMB Life. 2002 Sep;54(3):101-7. doi: 10.1080/15216540214541. IUBMB Life. 2002. PMID: 12489636 Review.
-
Quantitative Determination of Luminal and Abluminal Membrane Distributions of Transporters in Porcine Brain Capillaries by Plasma Membrane Fractionation and Quantitative Targeted Proteomics.J Pharm Sci. 2015 Sep;104(9):3060-8. doi: 10.1002/jps.24398. Epub 2015 Feb 20. J Pharm Sci. 2015. PMID: 25703048
Cited by
-
Advanced Immunolabeling Method for Optical Volumetric Imaging Reveals Dystrophic Neurites of Dopaminergic Neurons in Alzheimer's Disease Mouse Brain.Mol Neurobiol. 2024 Jul;61(7):3976-3999. doi: 10.1007/s12035-023-03823-9. Epub 2023 Dec 4. Mol Neurobiol. 2024. PMID: 38049707 Free PMC article.
-
Longitudinal evaluation of neuroinflammation and oxidative stress in a mouse model of Alzheimer disease using positron emission tomography.Alzheimers Res Ther. 2022 Jun 9;14(1):80. doi: 10.1186/s13195-022-01016-5. Alzheimers Res Ther. 2022. PMID: 35676734 Free PMC article.
-
Oral antibiotics reduce voluntary exercise behavior in athletic mice.Behav Processes. 2022 Jun;199:104650. doi: 10.1016/j.beproc.2022.104650. Epub 2022 Apr 30. Behav Processes. 2022. PMID: 35504410 Free PMC article.
-
To restrict or not to restrict? Practical considerations for optimizing dietary protein interactions on levodopa absorption in Parkinson's disease.NPJ Parkinsons Dis. 2023 Jun 24;9(1):98. doi: 10.1038/s41531-023-00541-w. NPJ Parkinsons Dis. 2023. PMID: 37355689 Free PMC article. Review.
-
Peripheral and Central Impact of Methionine Source and Level on Growth Performance, Circulating Methionine Levels and Metabolism in Broiler Chickens.Animals (Basel). 2023 Jun 12;13(12):1961. doi: 10.3390/ani13121961. Animals (Basel). 2023. PMID: 37370471 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

