Genome-wide screens identify Toxoplasma gondii determinants of parasite fitness in IFNγ-activated murine macrophages
- PMID: 33067458
- PMCID: PMC7567896
- DOI: 10.1038/s41467-020-18991-8
Genome-wide screens identify Toxoplasma gondii determinants of parasite fitness in IFNγ-activated murine macrophages
Abstract
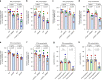
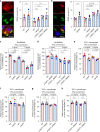
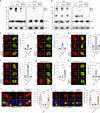
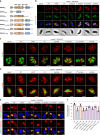
Macrophages play an essential role in the early immune response against Toxoplasma and are the cell type preferentially infected by the parasite in vivo. Interferon gamma (IFNγ) elicits a variety of anti-Toxoplasma activities in macrophages. Using a genome-wide CRISPR screen we identify 353 Toxoplasma genes that determine parasite fitness in naїve or IFNγ-activated murine macrophages, seven of which are further confirmed. We show that one of these genes encodes dense granule protein GRA45, which has a chaperone-like domain, is critical for correct localization of GRAs into the PVM and secretion of GRA effectors into the host cytoplasm. Parasites lacking GRA45 are more susceptible to IFNγ-mediated growth inhibition and have reduced virulence in mice. Together, we identify and characterize an important chaperone-like GRA in Toxoplasma and provide a resource for the community to further explore the function of Toxoplasma genes that determine fitness in IFNγ-activated macrophages.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
A combination of four Toxoplasma gondii nuclear-targeted effectors protects against interferon gamma-driven human host cell death.mBio. 2024 Oct 16;15(10):e0212424. doi: 10.1128/mbio.02124-24. Epub 2024 Sep 18. mBio. 2024. PMID: 39292011 Free PMC article.
-
Toxoplasma gondii Parasitophorous Vacuole Membrane-Associated Dense Granule Proteins Orchestrate Chronic Infection and GRA12 Underpins Resistance to Host Gamma Interferon.mBio. 2019 Jul 2;10(4):e00589-19. doi: 10.1128/mBio.00589-19. mBio. 2019. PMID: 31266861 Free PMC article.
-
Secreted Toxoplasma gondii molecules interfere with expression of MHC-II in interferon gamma-activated macrophages.Int J Parasitol. 2015 Apr;45(5):319-32. doi: 10.1016/j.ijpara.2015.01.003. Epub 2015 Feb 24. Int J Parasitol. 2015. PMID: 25720921
-
GRA proteins of Toxoplasma gondii: maintenance of host-parasite interactions across the parasitophorous vacuolar membrane.Korean J Parasitol. 2009 Oct;47 Suppl(Suppl):S29-37. doi: 10.3347/kjp.2009.47.S.S29. Korean J Parasitol. 2009. PMID: 19885333 Free PMC article. Review.
-
Toxoplasma gondii effectors are master regulators of the inflammatory response.Trends Parasitol. 2011 Nov;27(11):487-95. doi: 10.1016/j.pt.2011.08.001. Epub 2011 Sep 3. Trends Parasitol. 2011. PMID: 21893432 Free PMC article. Review.
Cited by
-
High-throughput CRISPR technology: a novel horizon for solid organ transplantation.Front Immunol. 2024 Jan 4;14:1295523. doi: 10.3389/fimmu.2023.1295523. eCollection 2023. Front Immunol. 2024. PMID: 38239344 Free PMC article. Review.
-
A heterotrimeric complex of Toxoplasma proteins promotes parasite survival in interferon gamma-stimulated human cells.PLoS Biol. 2023 Jul 17;21(7):e3002202. doi: 10.1371/journal.pbio.3002202. eCollection 2023 Jul. PLoS Biol. 2023. PMID: 37459303 Free PMC article.
-
A Combination of Four Nuclear Targeted Effectors Protects Toxoplasma Against Interferon Gamma Driven Human Host Cell Death During Acute Infection.bioRxiv [Preprint]. 2023 Dec 25:2023.12.24.573224. doi: 10.1101/2023.12.24.573224. bioRxiv. 2023. Update in: mBio. 2024 Oct 16;15(10):e0212424. doi: 10.1128/mbio.02124-24. PMID: 38234811 Free PMC article. Updated. Preprint.
-
CRISPR-based functional profiling of the Toxoplasma gondii genome during acute murine infection.Nat Microbiol. 2024 Sep;9(9):2323-2343. doi: 10.1038/s41564-024-01754-2. Epub 2024 Jul 8. Nat Microbiol. 2024. PMID: 38977907
-
Host cell proteins modulated upon Toxoplasma infection identified using proteomic approaches: a molecular rationale.Parasitol Res. 2022 Jul;121(7):1853-1865. doi: 10.1007/s00436-022-07541-4. Epub 2022 May 13. Parasitol Res. 2022. PMID: 35552534 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

