Metformin enhances anti-mycobacterial responses by educating CD8+ T-cell immunometabolic circuits
- PMID: 33067434
- PMCID: PMC7567856
- DOI: 10.1038/s41467-020-19095-z
Metformin enhances anti-mycobacterial responses by educating CD8+ T-cell immunometabolic circuits
Abstract
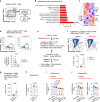
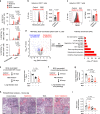
Patients with type 2 diabetes (T2D) have a lower risk of Mycobacterium tuberculosis infection, progression from infection to tuberculosis (TB) disease, TB morality and TB recurrence, when being treated with metformin. However, a detailed mechanistic understanding of these protective effects is lacking. Here, we use mass cytometry to show that metformin treatment expands a population of memory-like antigen-inexperienced CD8+CXCR3+ T cells in naive mice, and in healthy individuals and patients with T2D. Metformin-educated CD8+ T cells have increased (i) mitochondrial mass, oxidative phosphorylation, and fatty acid oxidation; (ii) survival capacity; and (iii) anti-mycobacterial properties. CD8+ T cells from Cxcr3-/- mice do not exhibit this metformin-mediated metabolic programming. In BCG-vaccinated mice and guinea pigs, metformin enhances immunogenicity and protective efficacy against M. tuberculosis challenge. Collectively, these results demonstrate an important function of CD8+ T cells in metformin-derived host metabolic-fitness towards M. tuberculosis infection.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Attrition of T-cell functions and simultaneous upregulation of inhibitory markers correspond with the waning of BCG-induced protection against tuberculosis in mice.PLoS One. 2014 Nov 24;9(11):e113951. doi: 10.1371/journal.pone.0113951. eCollection 2014. PLoS One. 2014. PMID: 25419982 Free PMC article.
-
Priming but not boosting with plasmid DNA encoding mycolyl-transferase Ag85A from Mycobacterium tuberculosis increases the survival time of Mycobacterium bovis BCG vaccinated mice against low dose intravenous challenge with M. tuberculosis H37Rv.Vaccine. 2006 Apr 12;24(16):3353-64. doi: 10.1016/j.vaccine.2005.12.066. Epub 2006 Feb 6. Vaccine. 2006. PMID: 16488518
-
A live attenuated BCG vaccine overexpressing multistage antigens Ag85B and HspX provides superior protection against Mycobacterium tuberculosis infection.Appl Microbiol Biotechnol. 2015 Dec;99(24):10587-95. doi: 10.1007/s00253-015-6962-x. Epub 2015 Sep 12. Appl Microbiol Biotechnol. 2015. PMID: 26363555
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
Is interferon-gamma the right marker for bacille Calmette-Guérin-induced immune protection? The missing link in our understanding of tuberculosis immunology.Clin Exp Immunol. 2012 Sep;169(3):213-9. doi: 10.1111/j.1365-2249.2012.04614.x. Clin Exp Immunol. 2012. PMID: 22861360 Free PMC article. Review.
Cited by
-
Metformin treatment rescues CD8+ T-cell response to immune checkpoint inhibitor therapy in mice with NAFLD.J Hepatol. 2022 Sep;77(3):748-760. doi: 10.1016/j.jhep.2022.03.010. Epub 2022 Apr 1. J Hepatol. 2022. PMID: 35378172 Free PMC article.
-
Gene expression signatures identify biologically and clinically distinct tuberculosis endotypes.Eur Respir J. 2022 Sep 15;60(3):2102263. doi: 10.1183/13993003.02263-2021. Print 2022 Sep. Eur Respir J. 2022. PMID: 35169026 Free PMC article.
-
Animal Models of Hepatocellular Carcinoma: Current Applications in Clinical Research.J Hepatocell Carcinoma. 2022 Dec 8;9:1263-1278. doi: 10.2147/JHC.S347946. eCollection 2022. J Hepatocell Carcinoma. 2022. PMID: 36523954 Free PMC article. Review.
-
Metformin promotes histone deacetylation of optineurin and suppresses tumour growth through autophagy inhibition in ocular melanoma.Clin Transl Med. 2022 Jan;12(1):e660. doi: 10.1002/ctm2.660. Clin Transl Med. 2022. PMID: 35075807 Free PMC article.
-
Immunologic, metabolic and genetic impact of diabetes on tuberculosis susceptibility.Front Immunol. 2023 Jan 23;14:1122255. doi: 10.3389/fimmu.2023.1122255. eCollection 2023. Front Immunol. 2023. PMID: 36756113 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

