Membrane Curvature, Trans-Membrane Area Asymmetry, Budding, Fission and Organelle Geometry
- PMID: 33066582
- PMCID: PMC7590041
- DOI: 10.3390/ijms21207594
Membrane Curvature, Trans-Membrane Area Asymmetry, Budding, Fission and Organelle Geometry
Abstract
In biology, the modern scientific fashion is to mostly study proteins. Much less attention is paid to lipids. However, lipids themselves are extremely important for the formation and functioning of cellular membrane organelles. Here, the role of the geometry of the lipid bilayer in regulation of organelle shape is analyzed. It is proposed that during rapid shape transition, the number of lipid heads and their size (i.e., due to the change in lipid head charge) inside lipid leaflets modulates the geometrical properties of organelles, in particular their membrane curvature. Insertion of proteins into a lipid bilayer and the shape of protein trans-membrane domains also affect the trans-membrane asymmetry between surface areas of luminal and cytosol leaflets of the membrane. In the cases where lipid molecules with a specific shape are not predominant, the shape of lipids (cylindrical, conical, or wedge-like) is less important for the regulation of membrane curvature, due to the flexibility of their acyl chains and their high ability to diffuse.
Keywords: COP; Golgi; budding; caveola; endosome; filopodia; membrane fission; mitochondria fusion; nuclear envelope; trans–membrane area asymmetry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
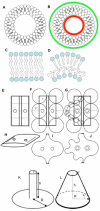
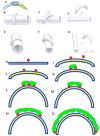
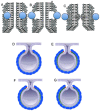
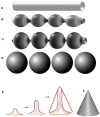

Similar articles
-
Trans-membrane area asymmetry controls the shape of cellular organelles.Int J Mol Sci. 2015 Mar 9;16(3):5299-333. doi: 10.3390/ijms16035299. Int J Mol Sci. 2015. PMID: 25761238 Free PMC article.
-
Protein-lipid interactions in membrane trafficking at the Golgi complex.Biochim Biophys Acta. 2004 Nov 3;1666(1-2):264-74. doi: 10.1016/j.bbamem.2004.07.002. Biochim Biophys Acta. 2004. PMID: 15519320 Review.
-
Shape transitions in lipid membranes and protein mediated vesicle fusion and fission.J Chem Phys. 2007 Mar 7;126(9):095102. doi: 10.1063/1.2483862. J Chem Phys. 2007. PMID: 17362130 Review.
-
Dynamics and instabilities of lipid bilayer membrane shapes.Adv Colloid Interface Sci. 2014 Jun;208:76-88. doi: 10.1016/j.cis.2014.01.004. Epub 2014 Jan 25. Adv Colloid Interface Sci. 2014. PMID: 24529968 Free PMC article. Review.
-
Budding and Fission of Nanovesicles Induced by Membrane Adsorption of Small Solutes.ACS Nano. 2021 Apr 27;15(4):7237-7248. doi: 10.1021/acsnano.1c00525. Epub 2021 Apr 5. ACS Nano. 2021. PMID: 33819031 Free PMC article.
Cited by
-
COVID-19 Biogenesis and Intracellular Transport.Int J Mol Sci. 2023 Feb 24;24(5):4523. doi: 10.3390/ijms24054523. Int J Mol Sci. 2023. PMID: 36901955 Free PMC article. Review.
-
The C10orf76-PI4KB axis orchestrates CERT-mediated ceramide trafficking to the distal Golgi.J Cell Biol. 2023 Jul 3;222(7):e202111069. doi: 10.1083/jcb.202111069. Epub 2023 May 17. J Cell Biol. 2023. PMID: 37195633 Free PMC article.
-
Curvature-based sorting of eight lipid types in asymmetric buckled plasma membrane models.Biophys J. 2022 Jun 7;121(11):2060-2068. doi: 10.1016/j.bpj.2022.05.002. Epub 2022 May 5. Biophys J. 2022. PMID: 35524412 Free PMC article.
-
LC-ESI-QTOF/MS characterization of antimicrobial compounds with their action mode extracted from vine tea (Ampelopsis grossedentata) leaves.Food Sci Nutr. 2022 Jan 19;10(2):422-435. doi: 10.1002/fsn3.2679. eCollection 2022 Feb. Food Sci Nutr. 2022. PMID: 35154679 Free PMC article.
-
The Diffusion Model of Intra-Golgi Transport Has Limited Power.Int J Mol Sci. 2023 Jan 10;24(2):1375. doi: 10.3390/ijms24021375. Int J Mol Sci. 2023. PMID: 36674888 Free PMC article.
References
-
- Derganc J., Mironov A.A., Svetina S. The geometry of organelles of the secretory pathway. In: Mironov A.A., Pavelka M., editors. The Golgi Apparatus. Springer; Berlin/Heidelberg, Germany: 2008. pp. 314–330.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

